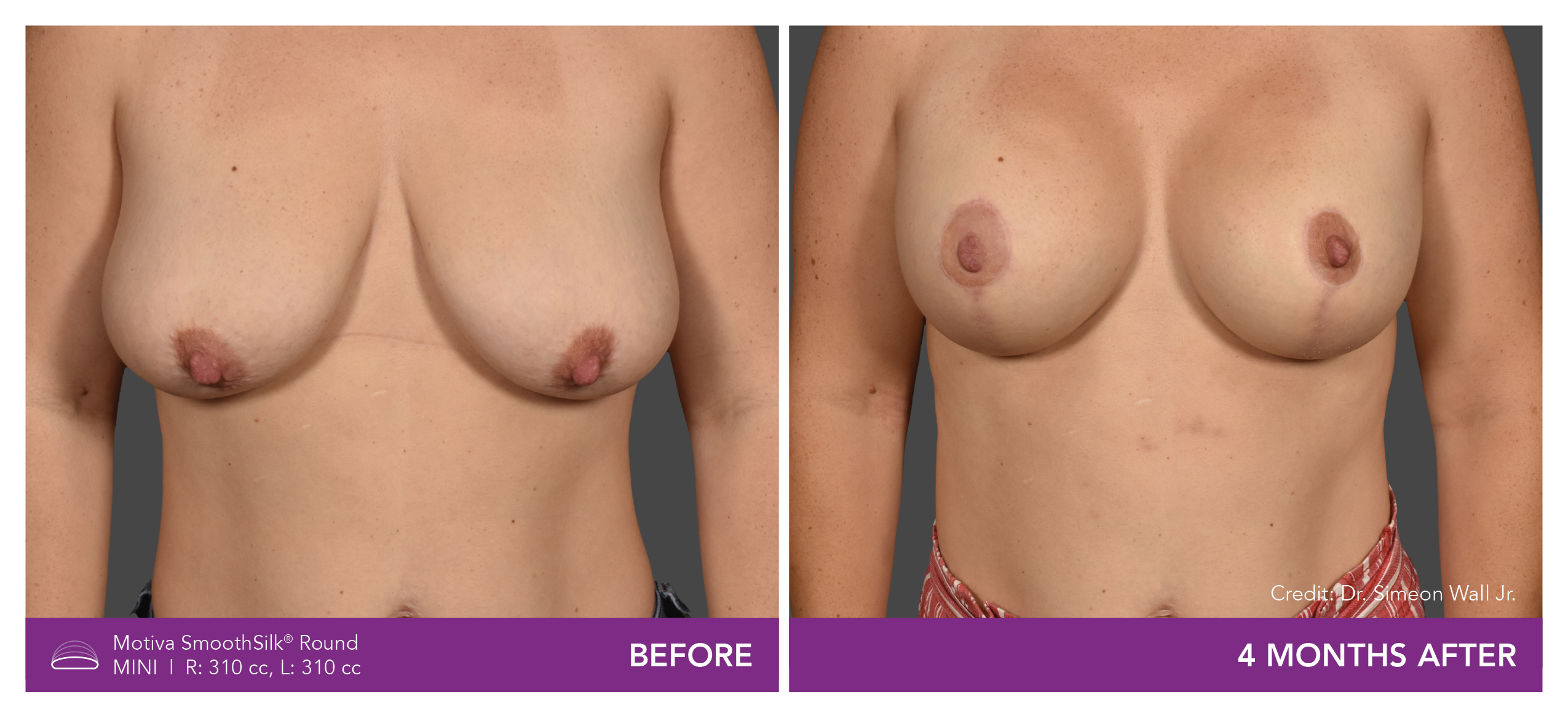
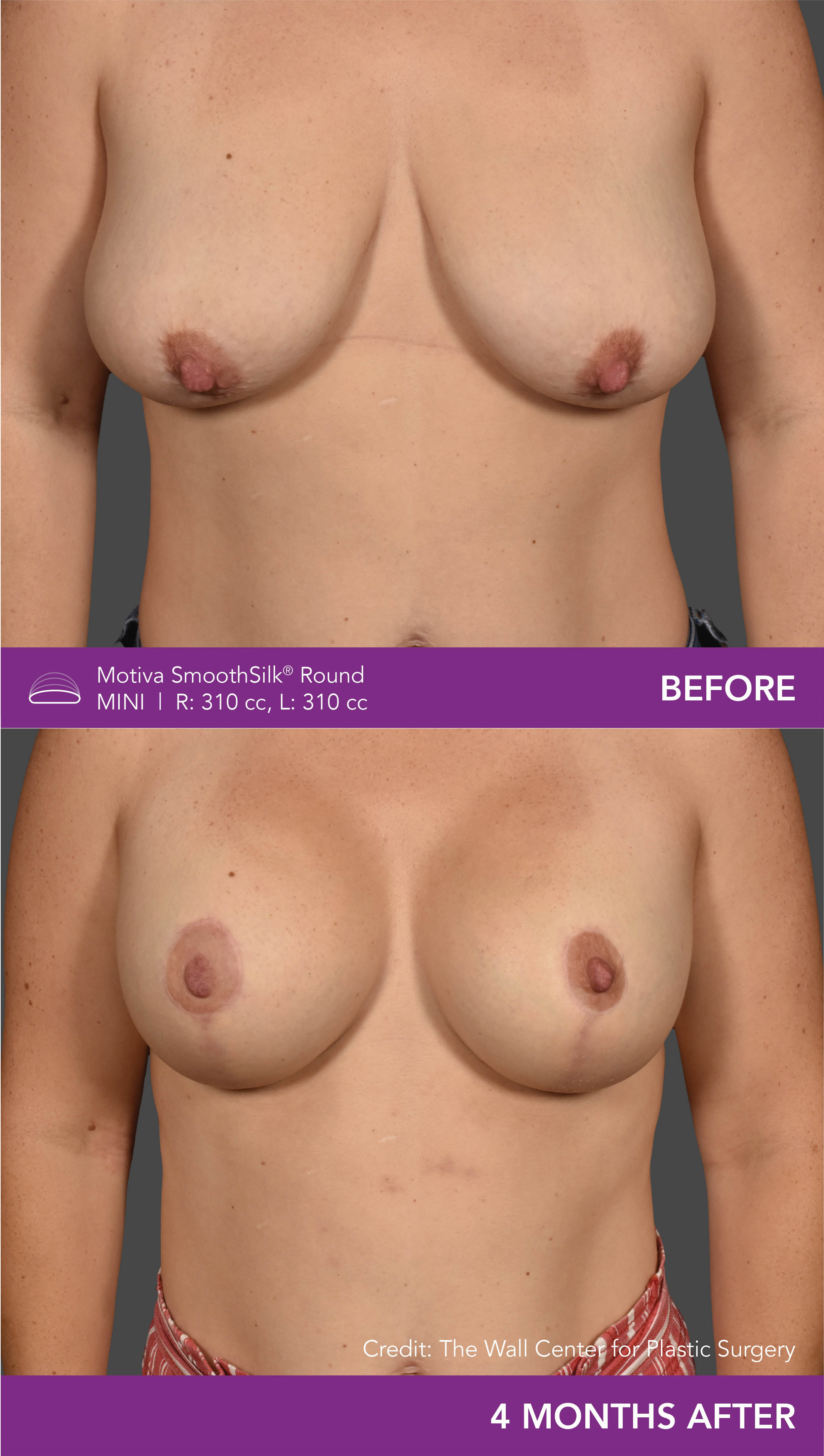
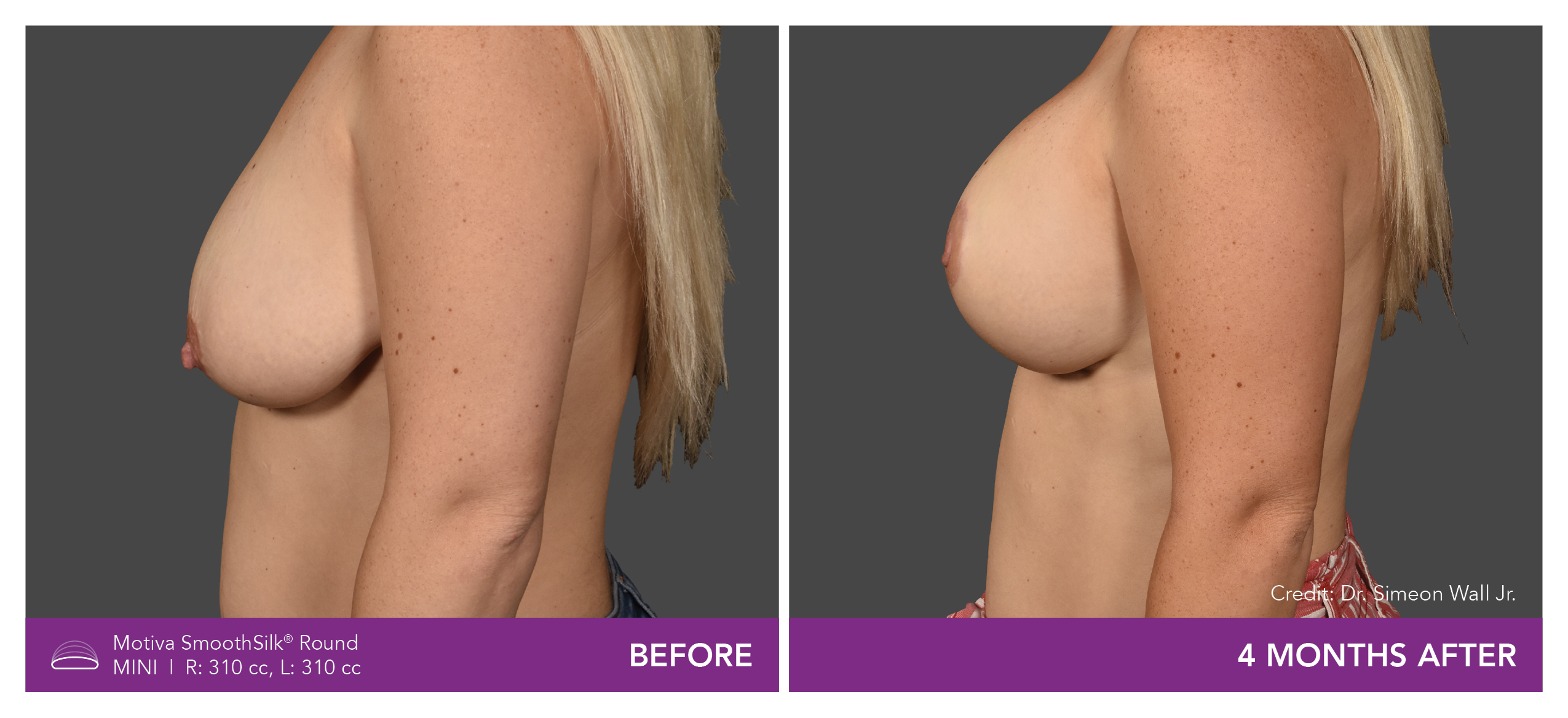
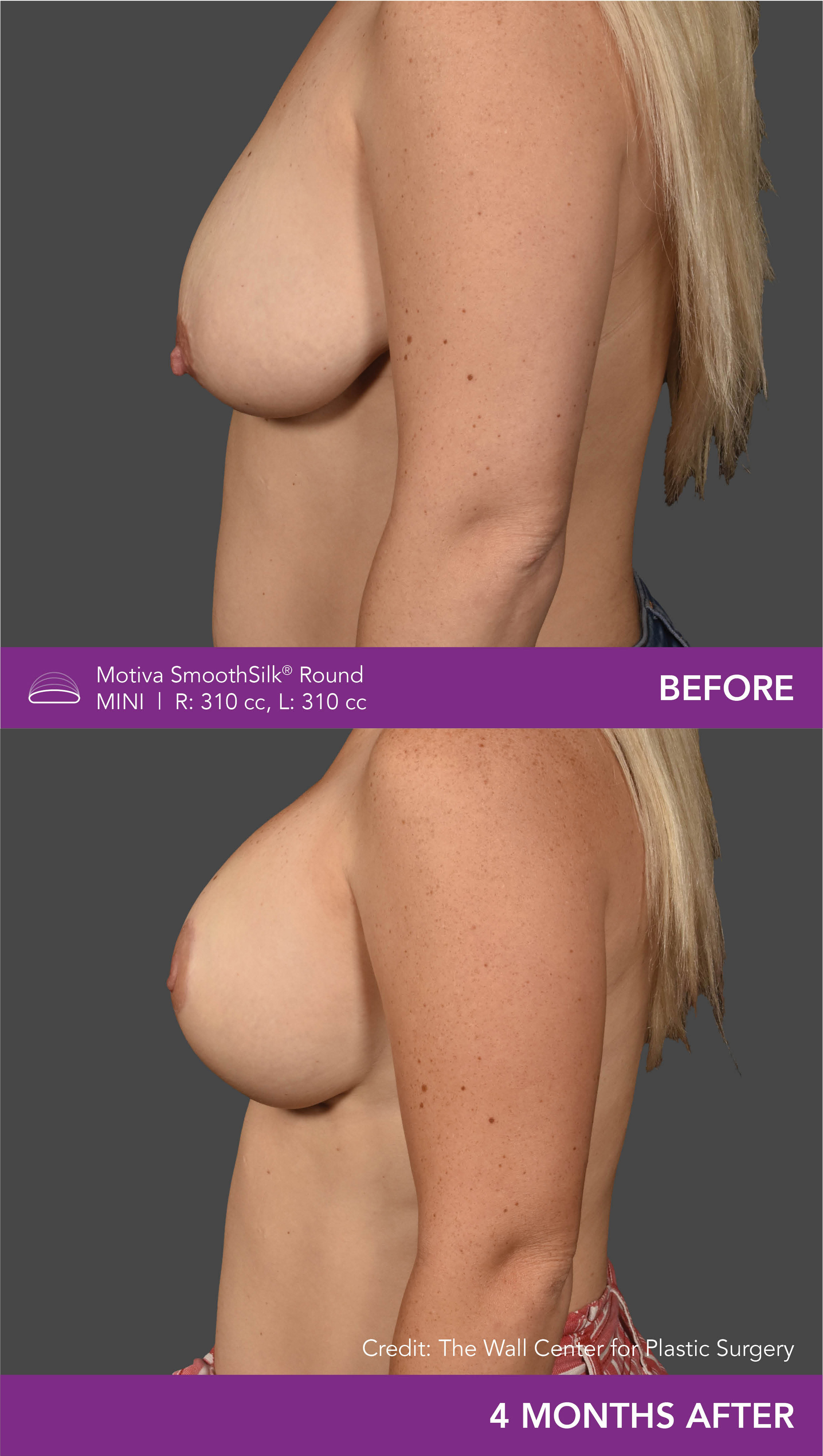
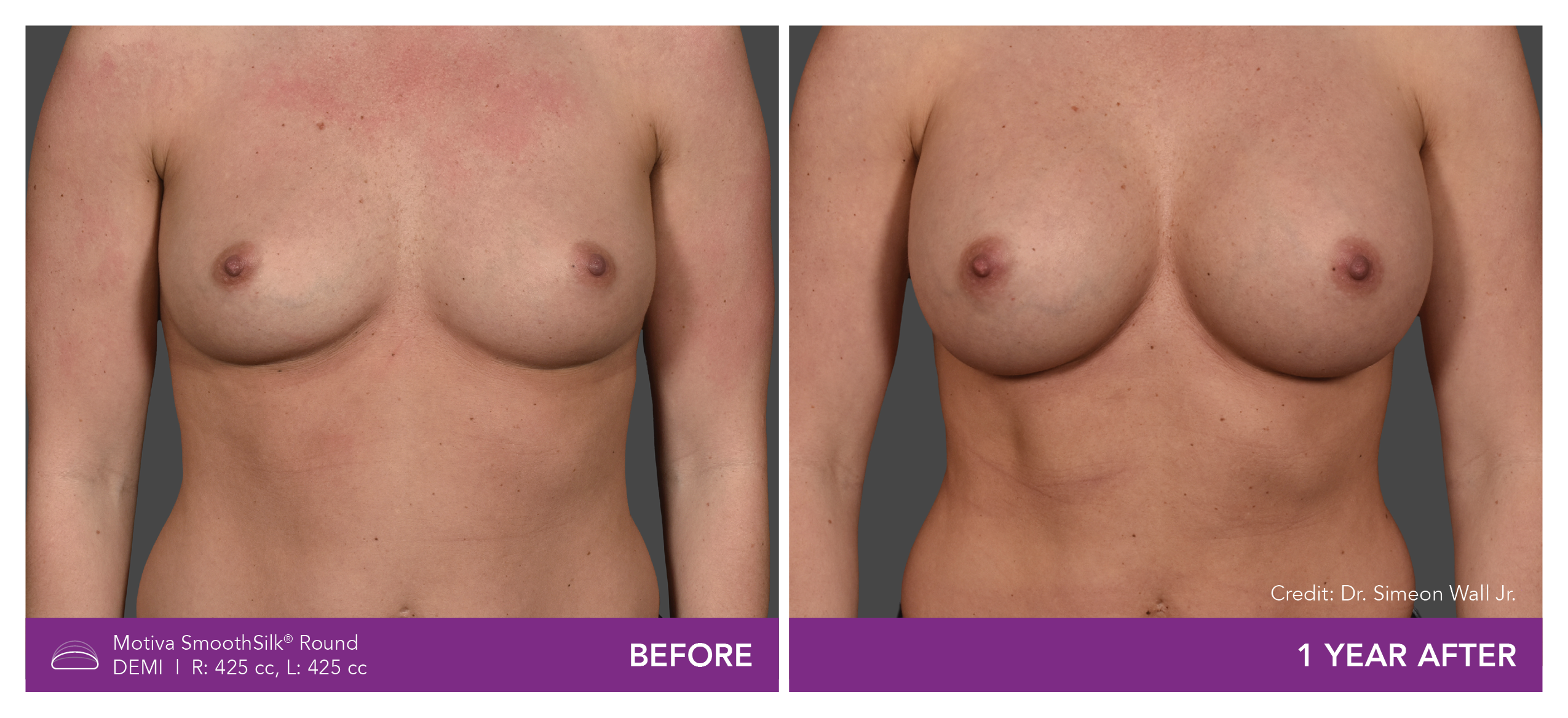
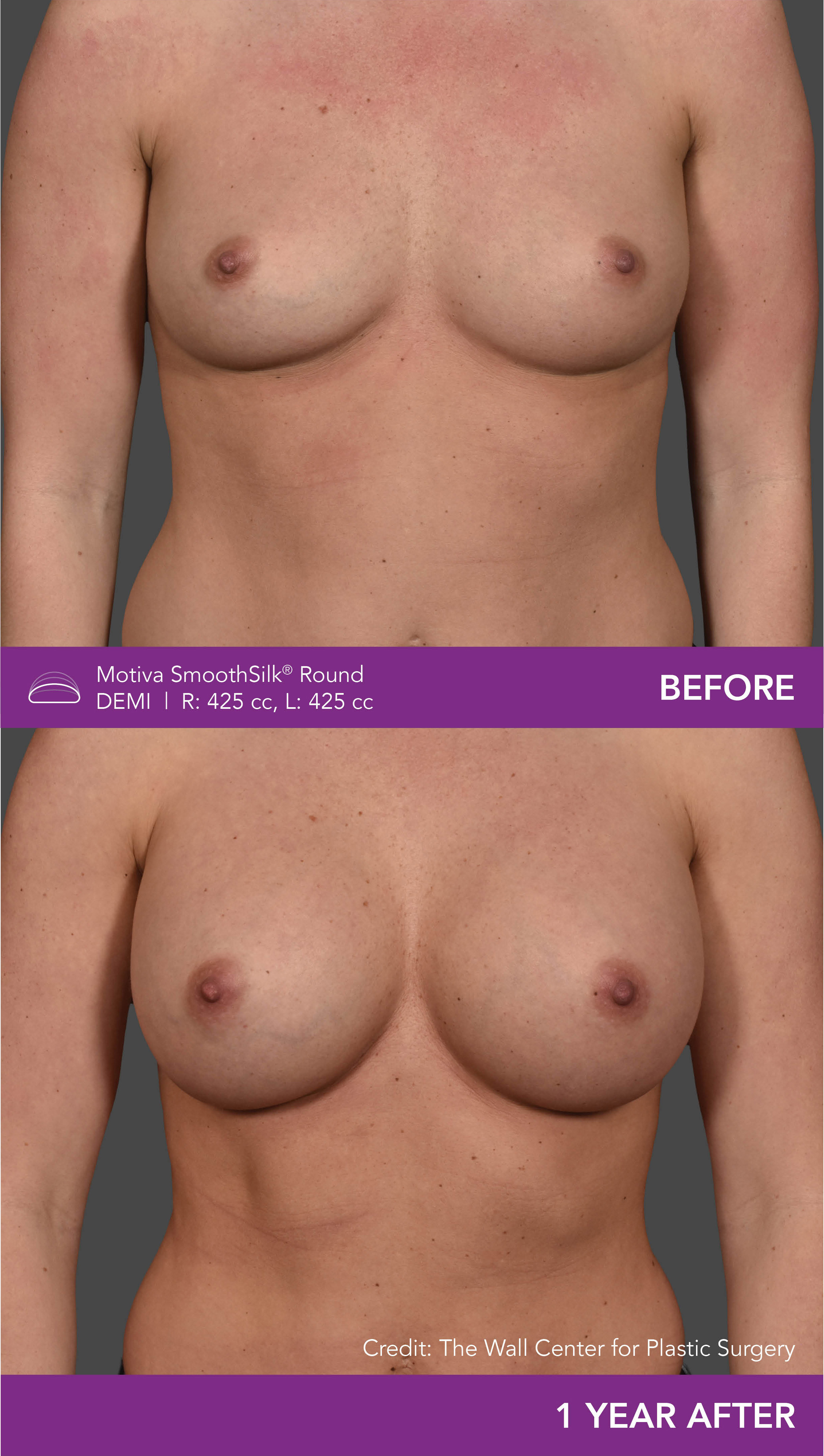
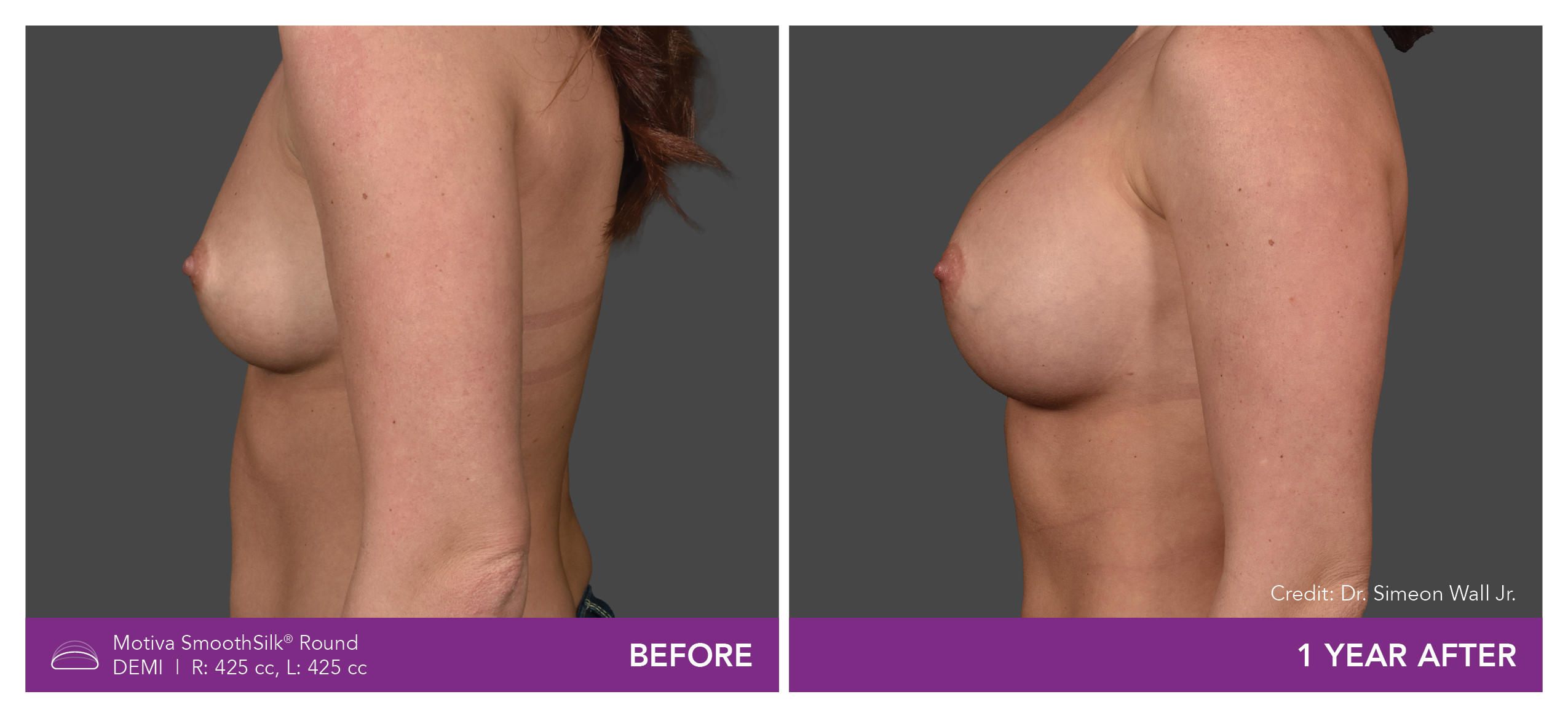
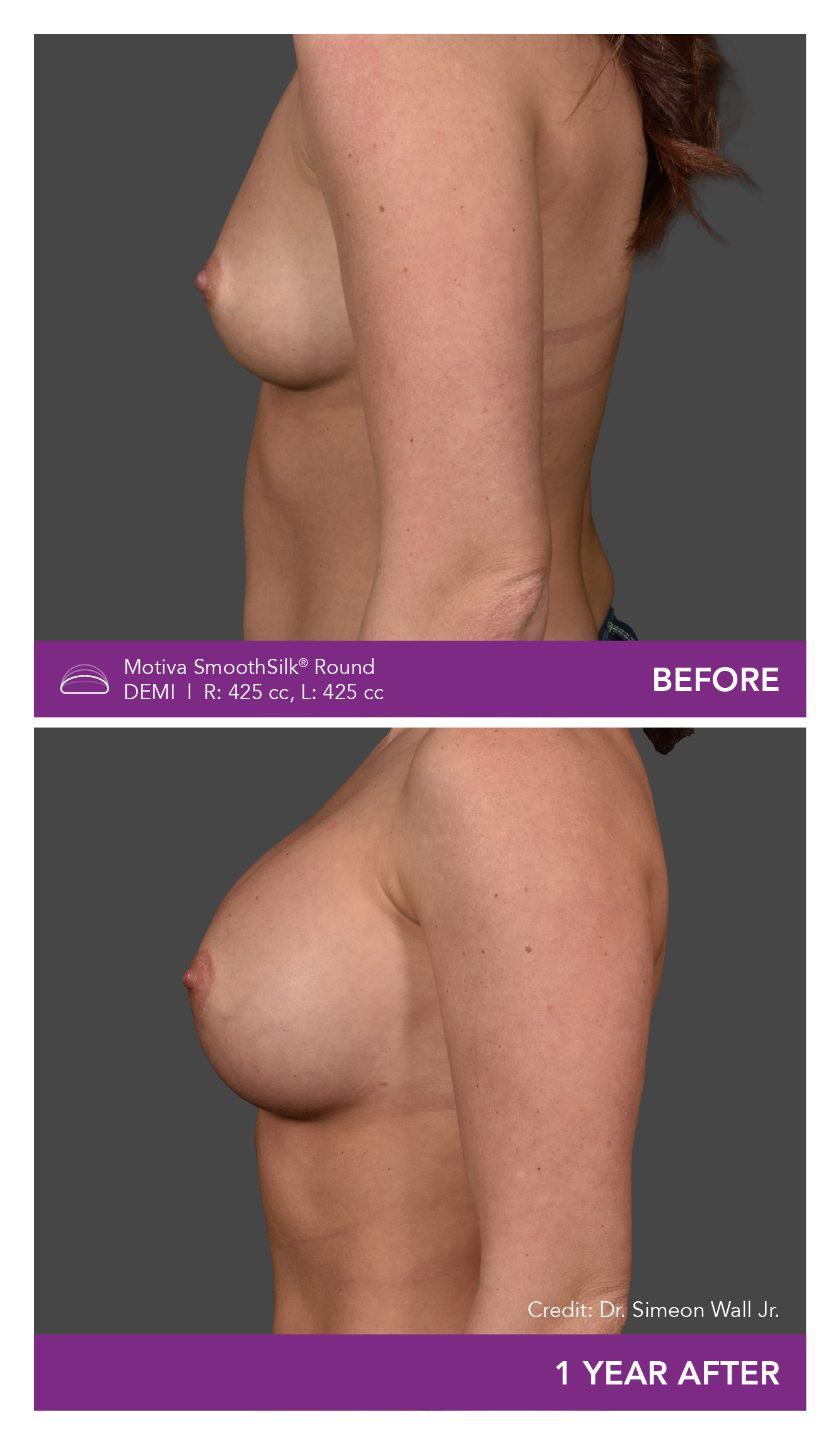
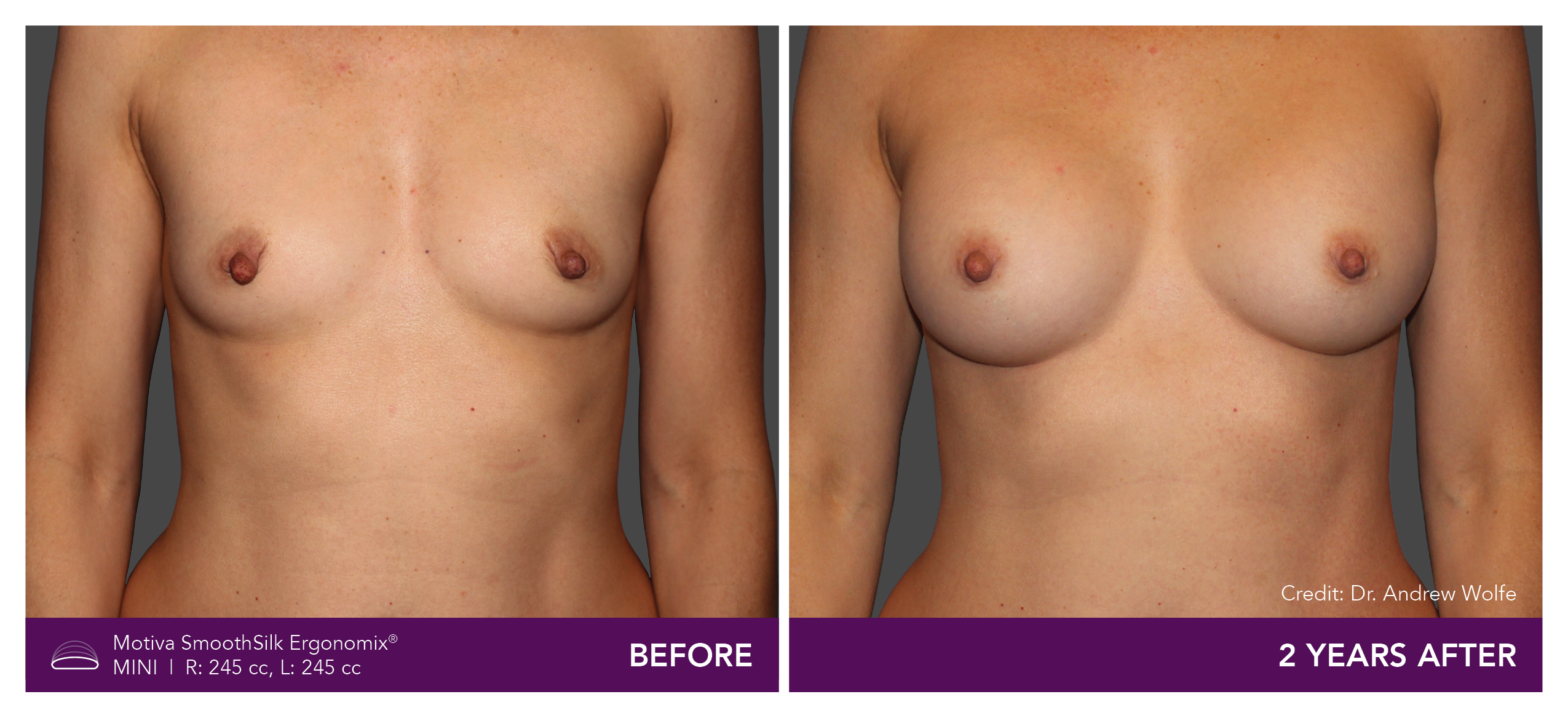
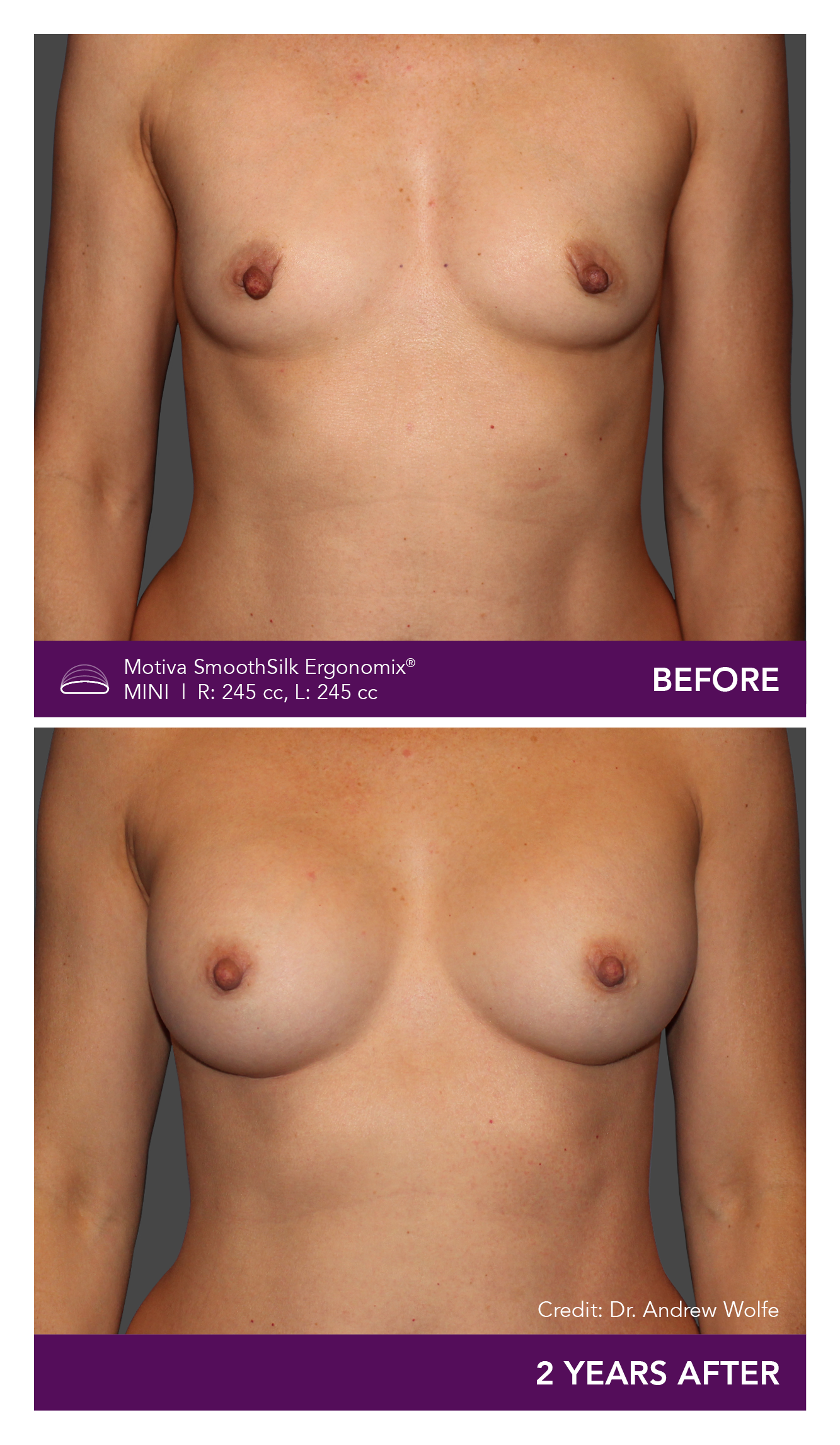
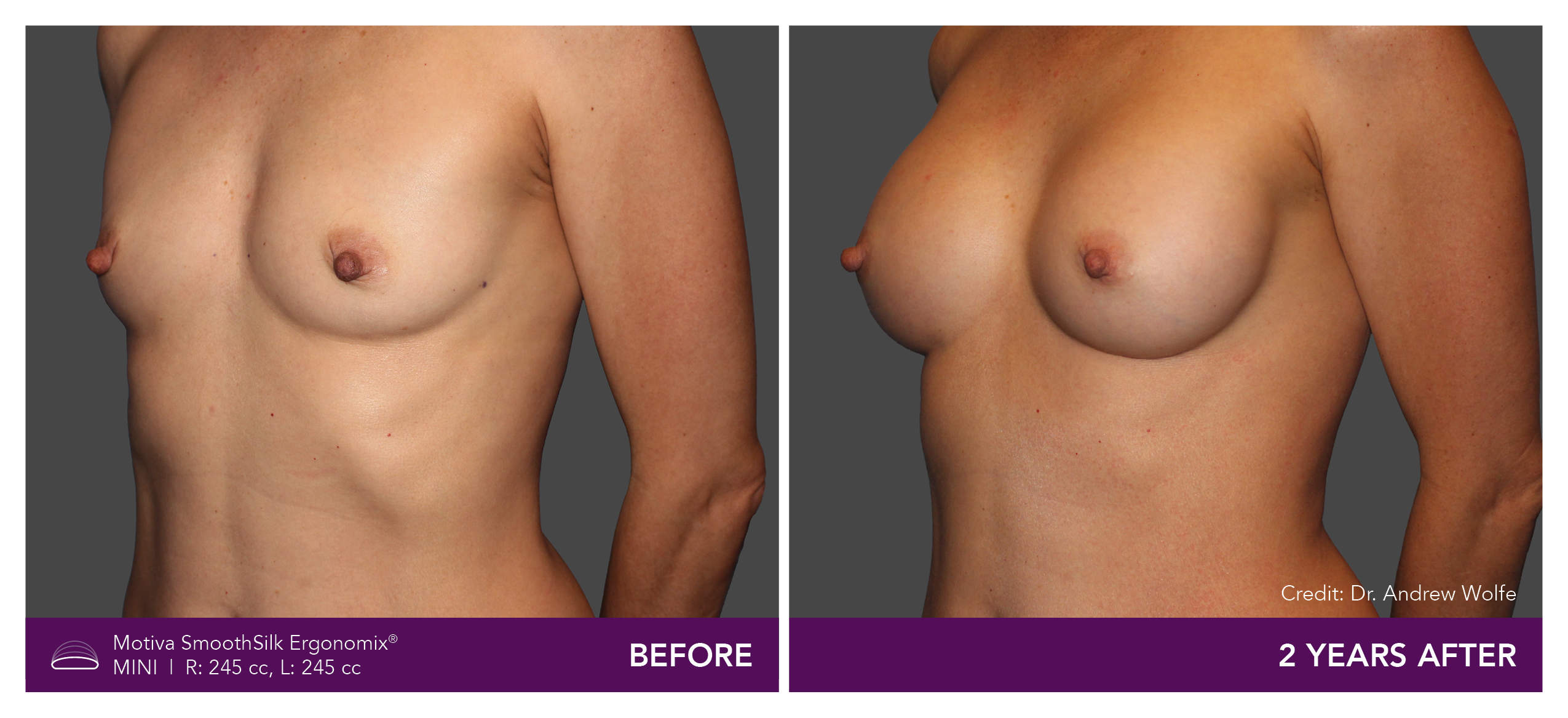
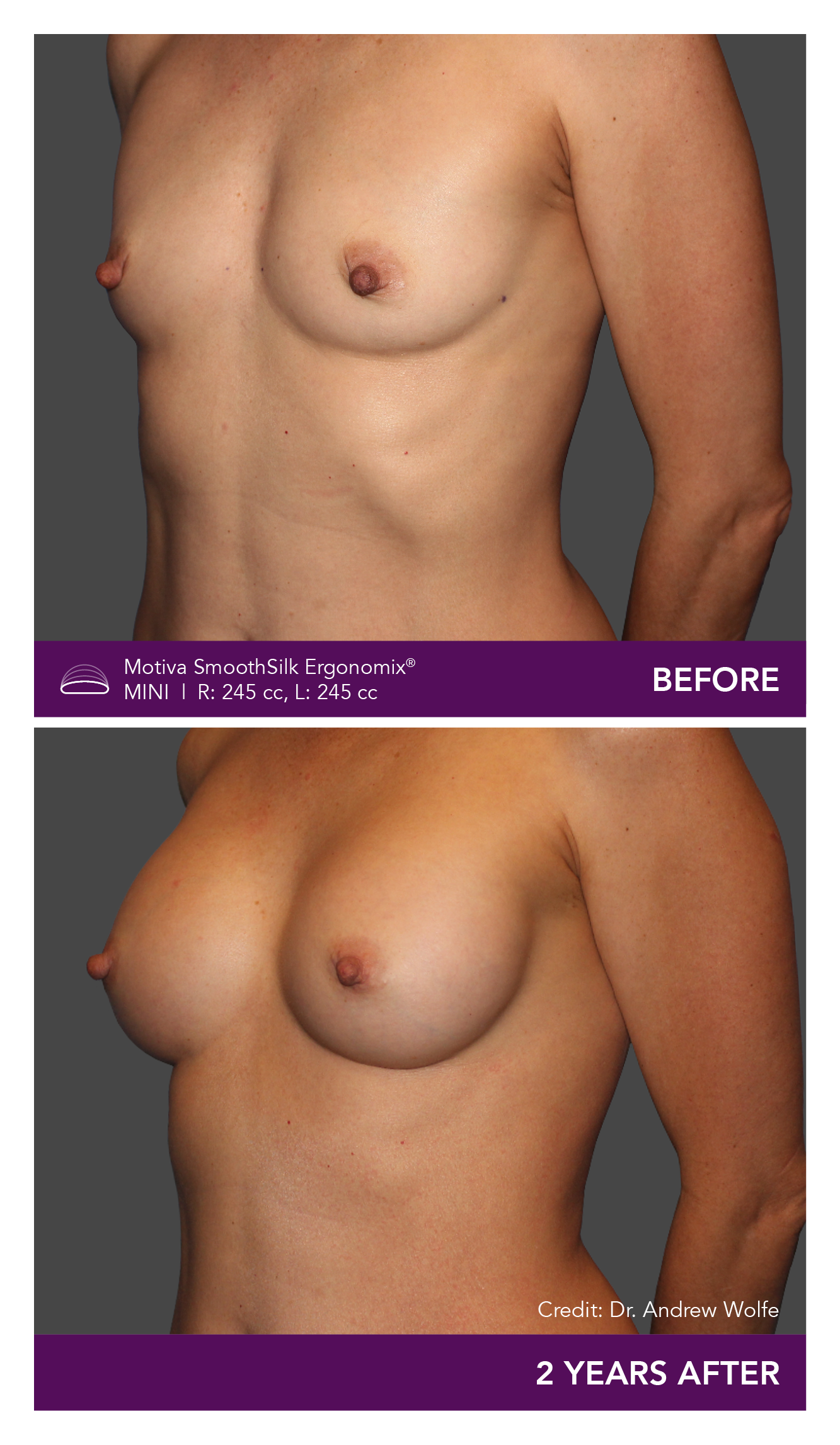
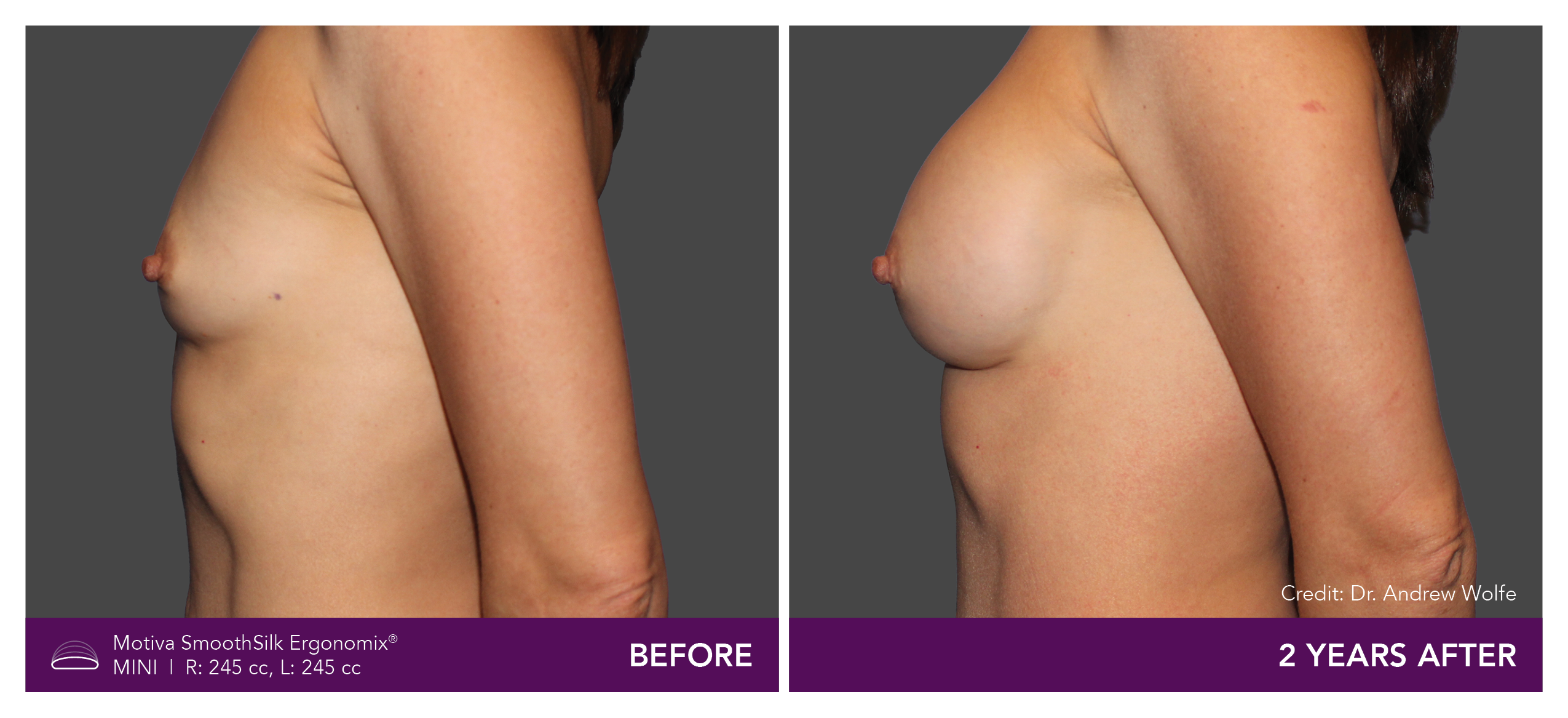
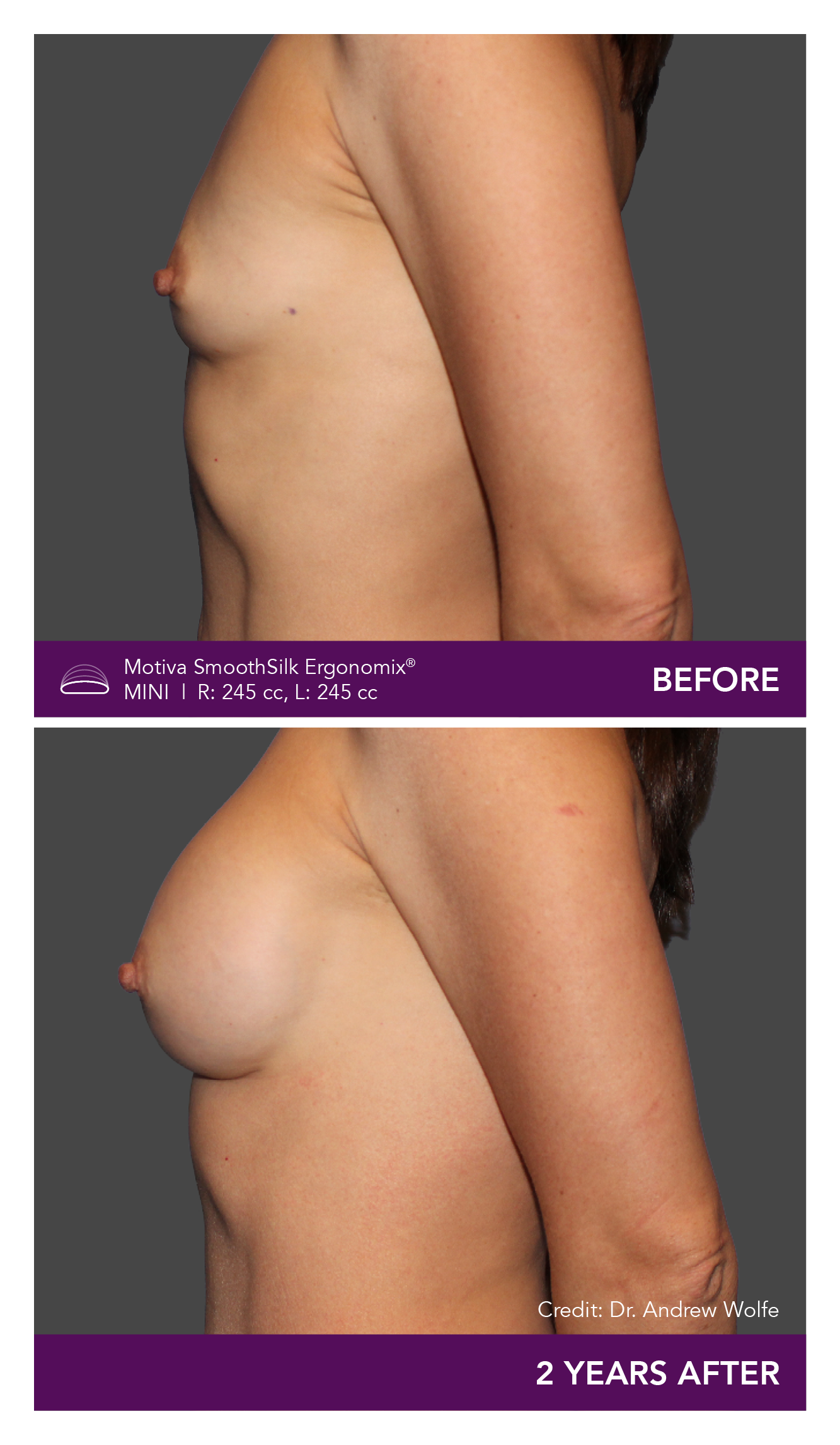
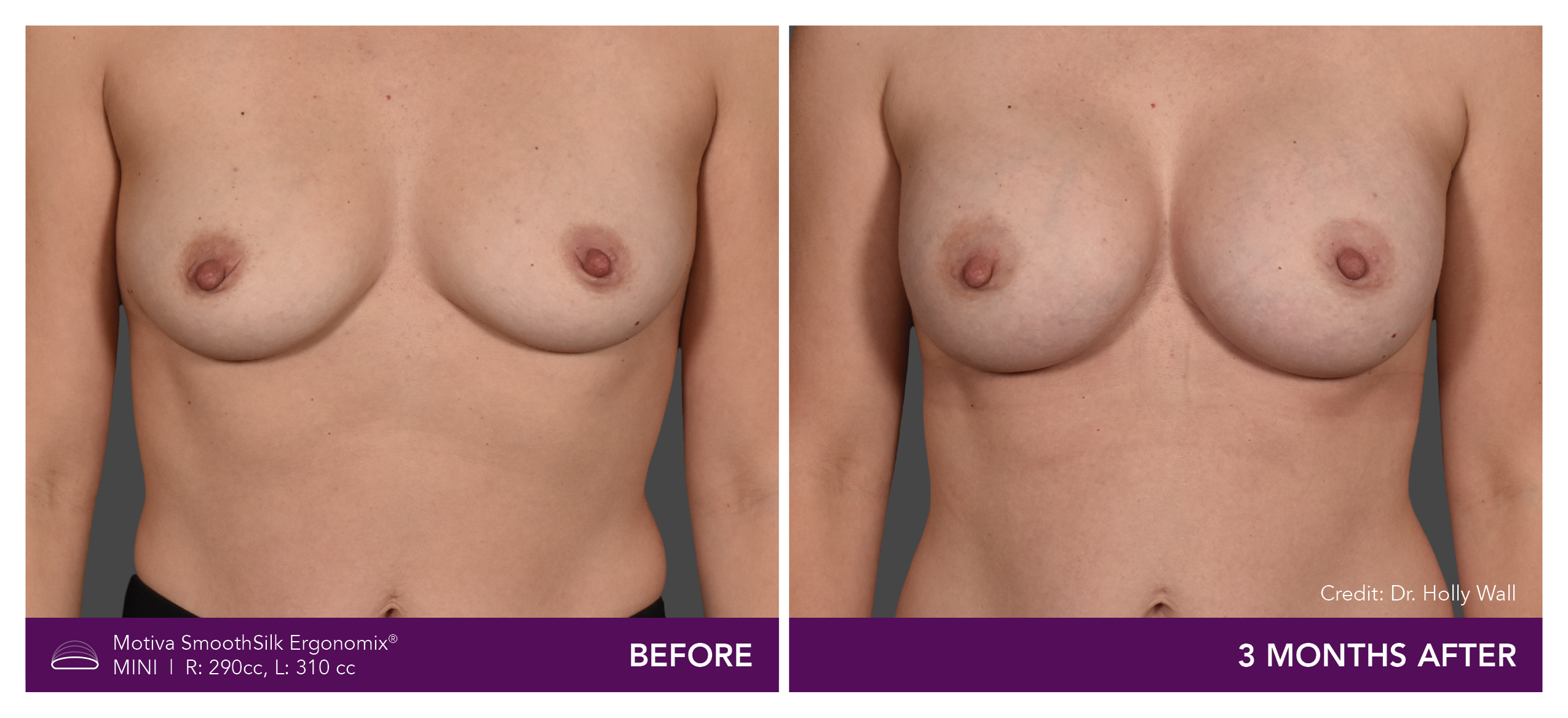
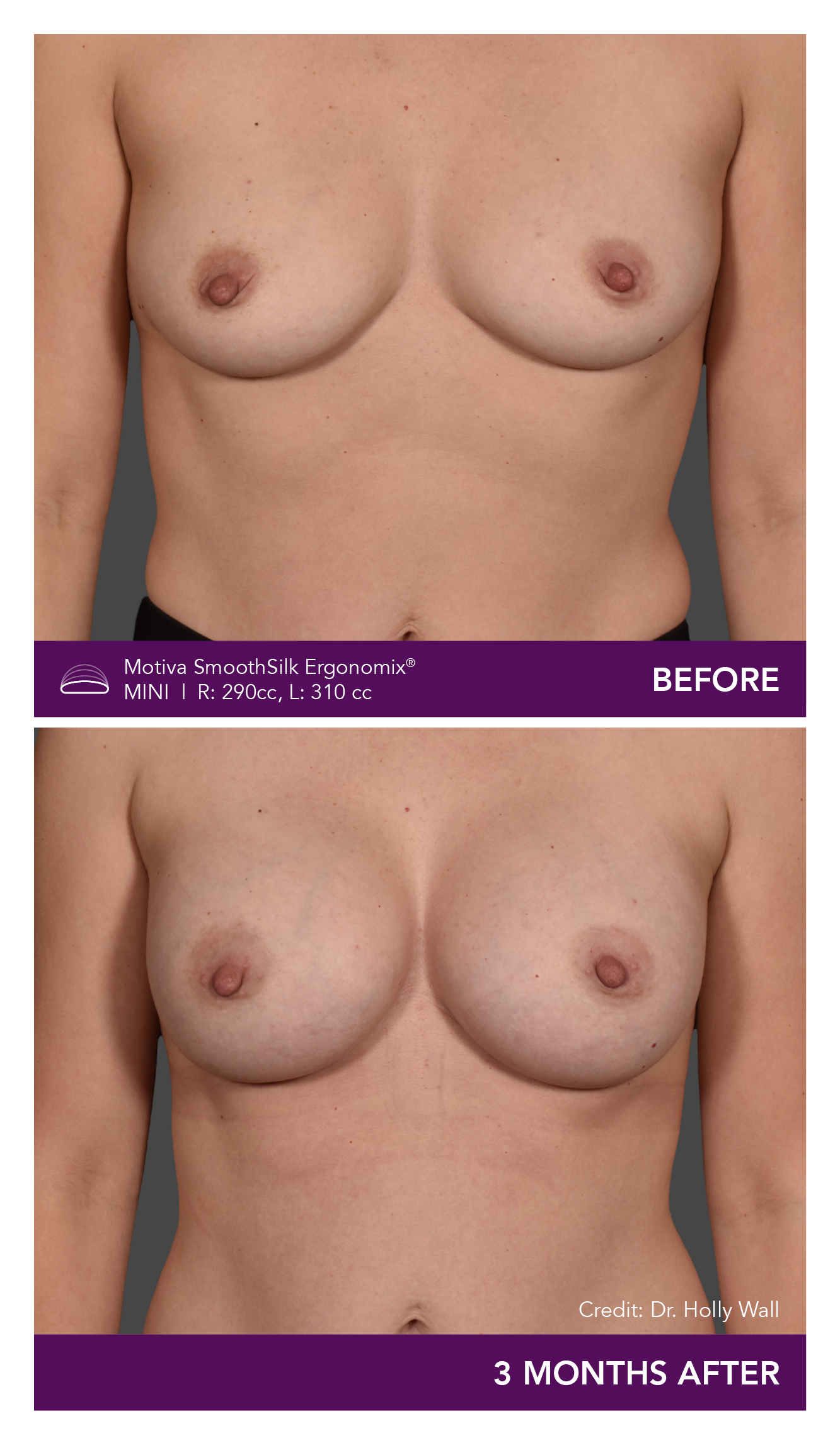
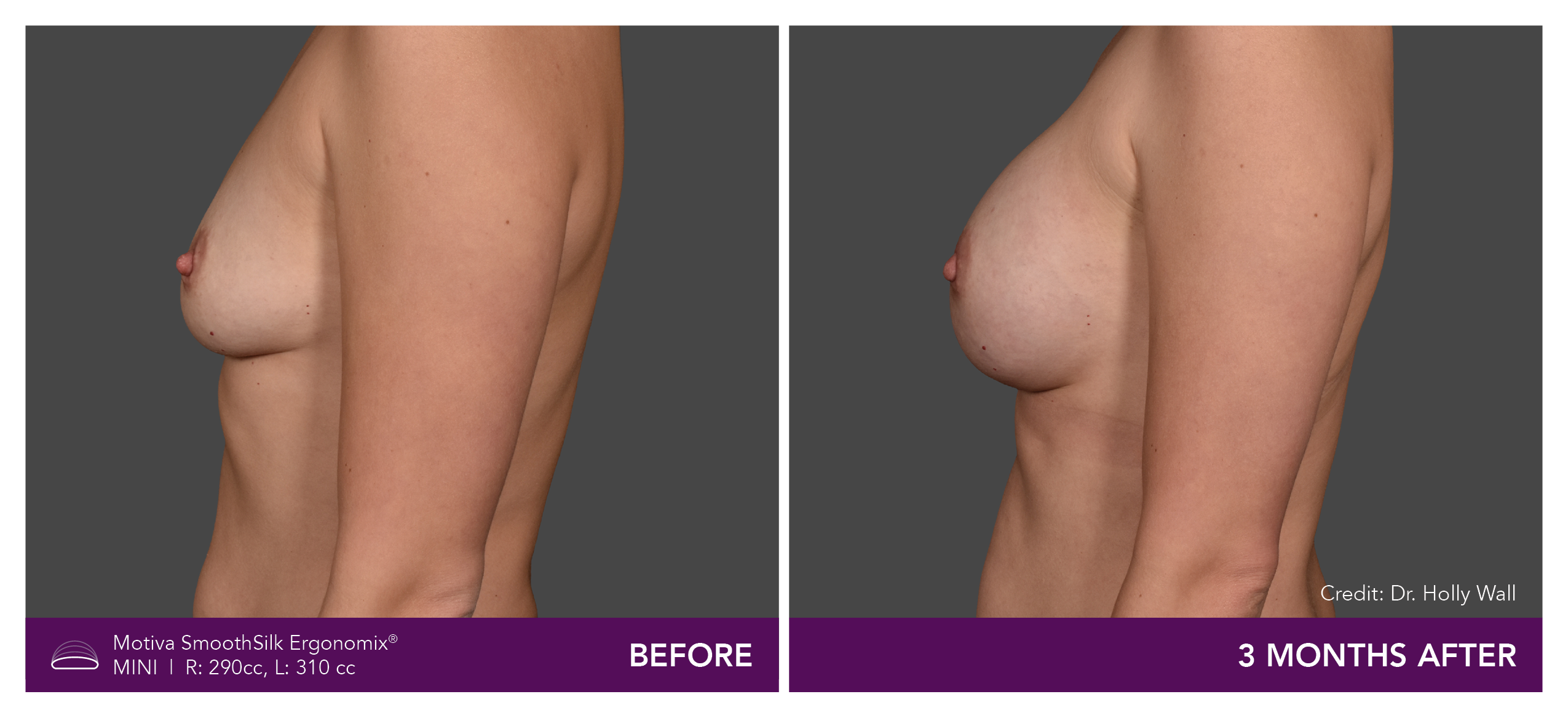
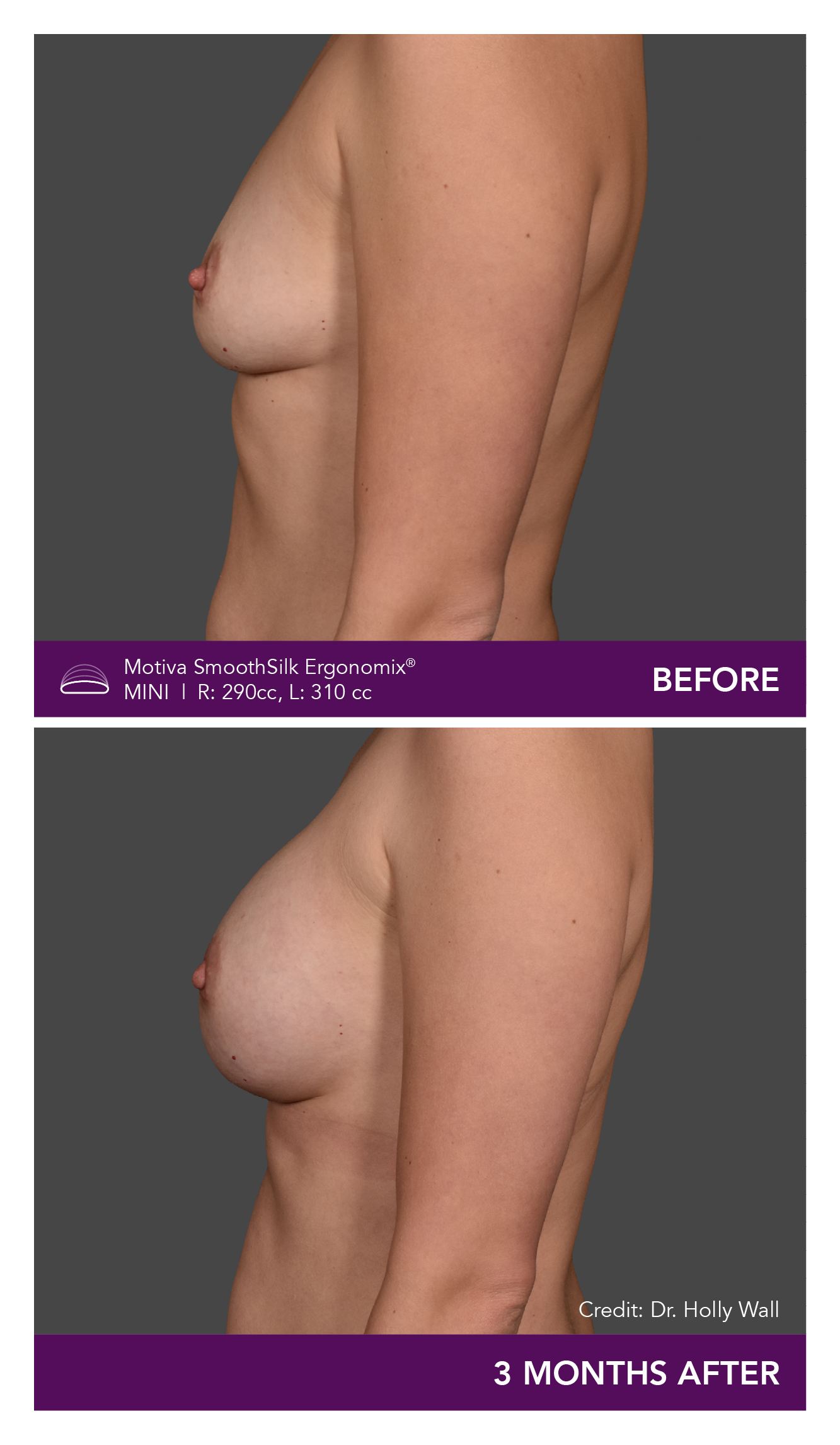
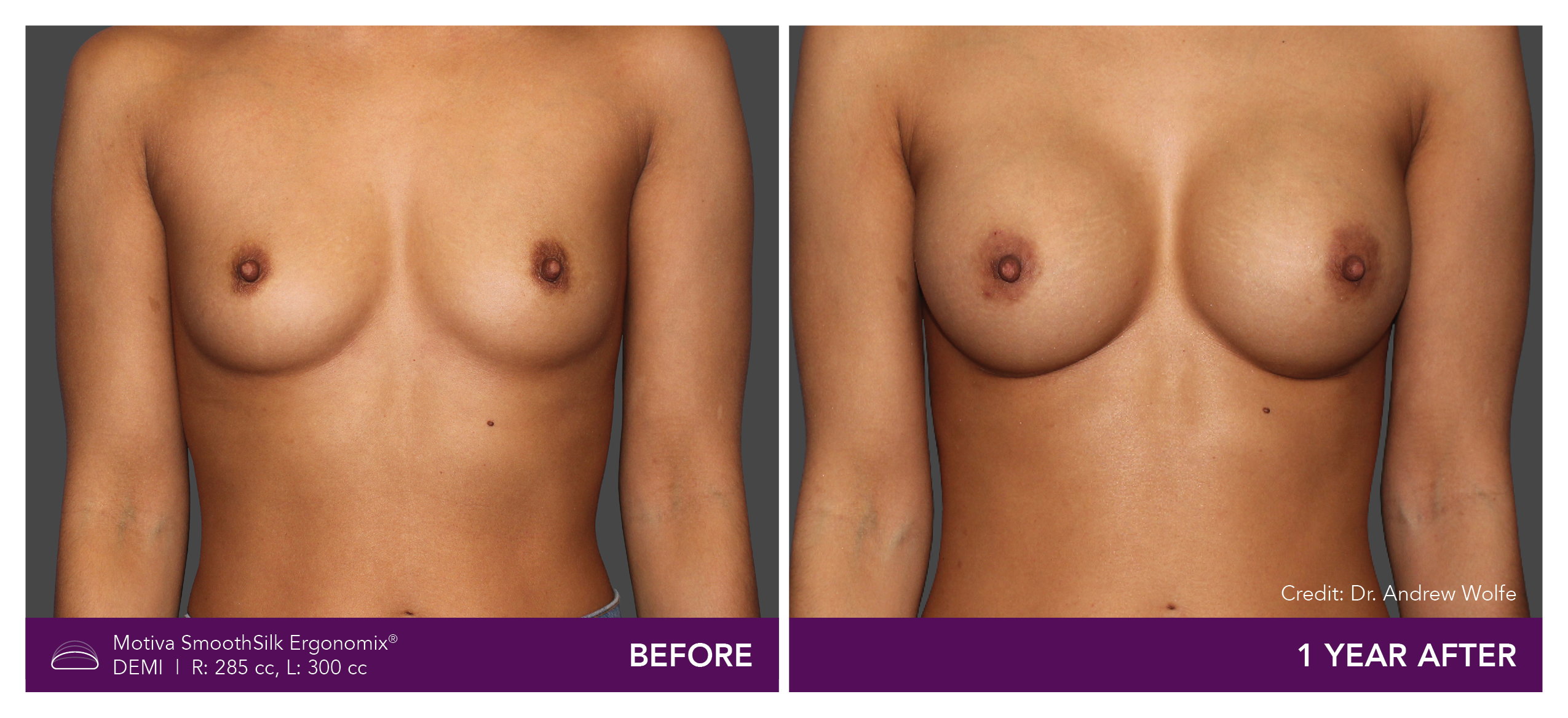
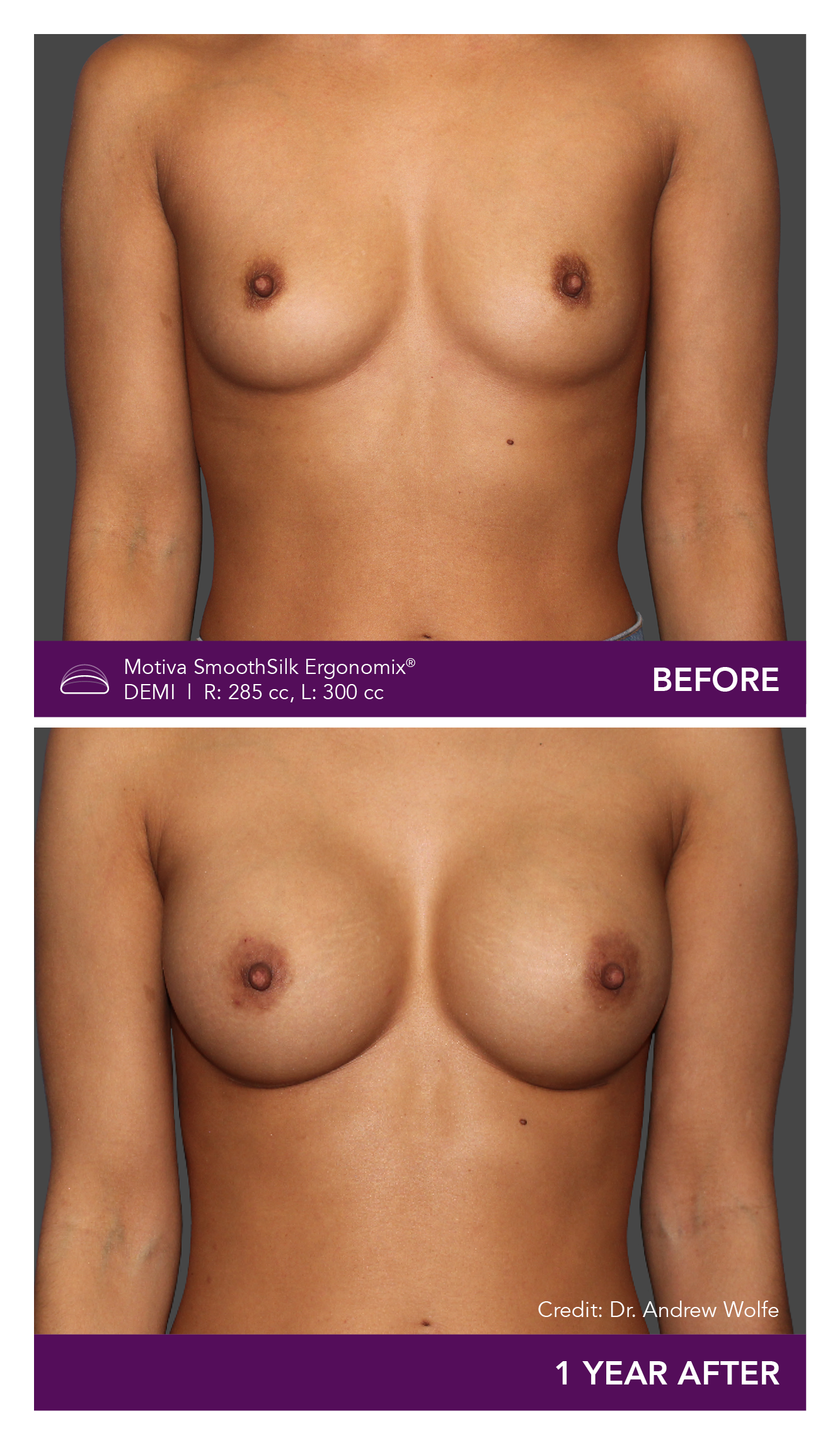
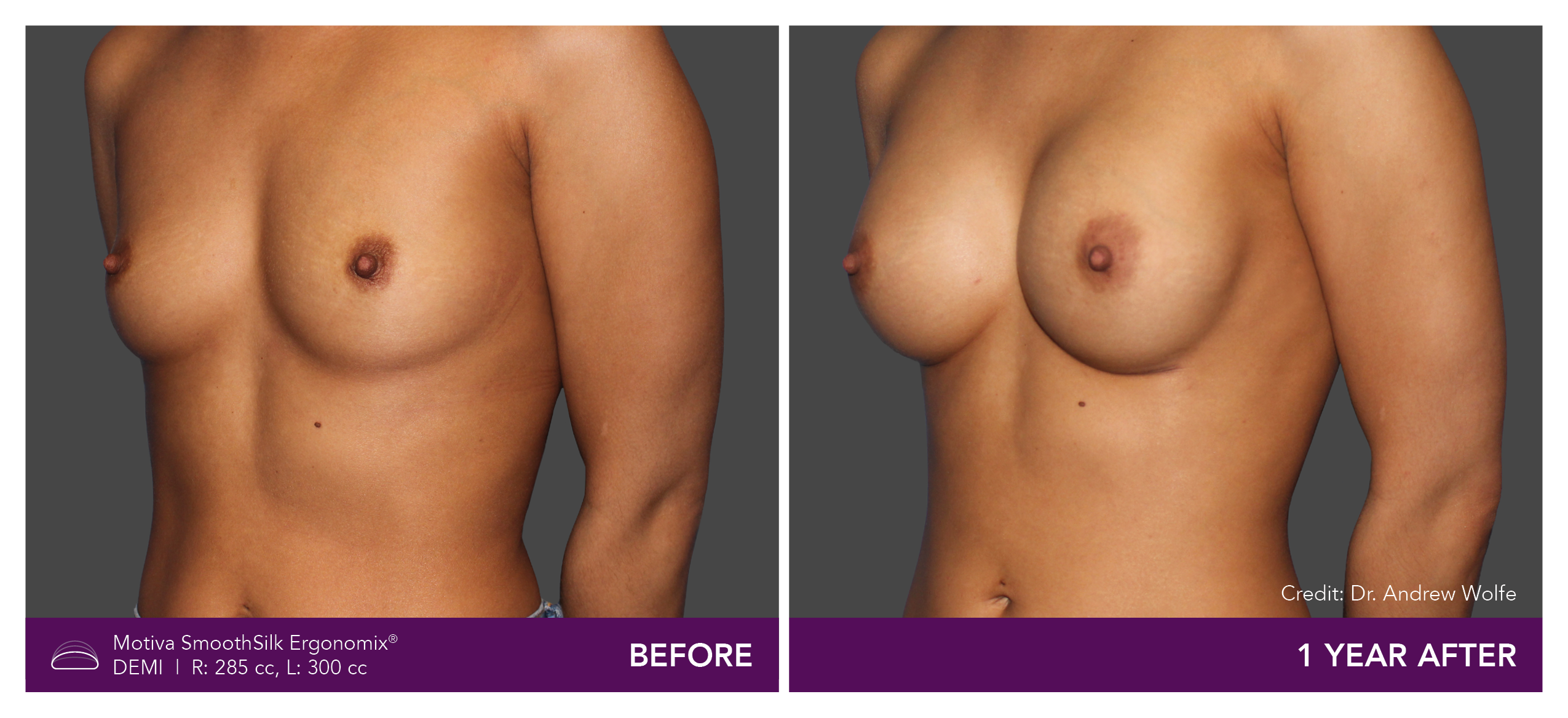
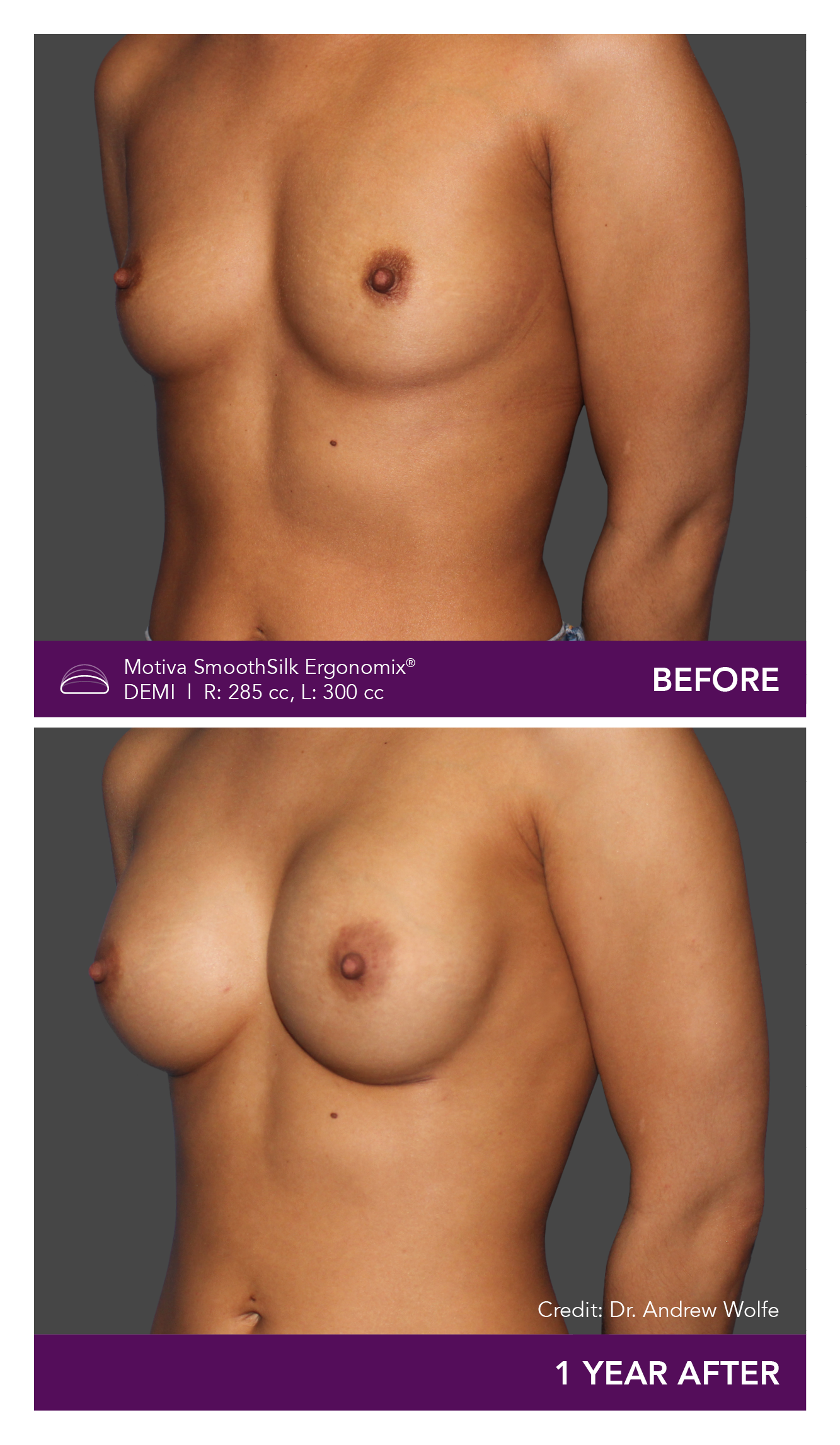
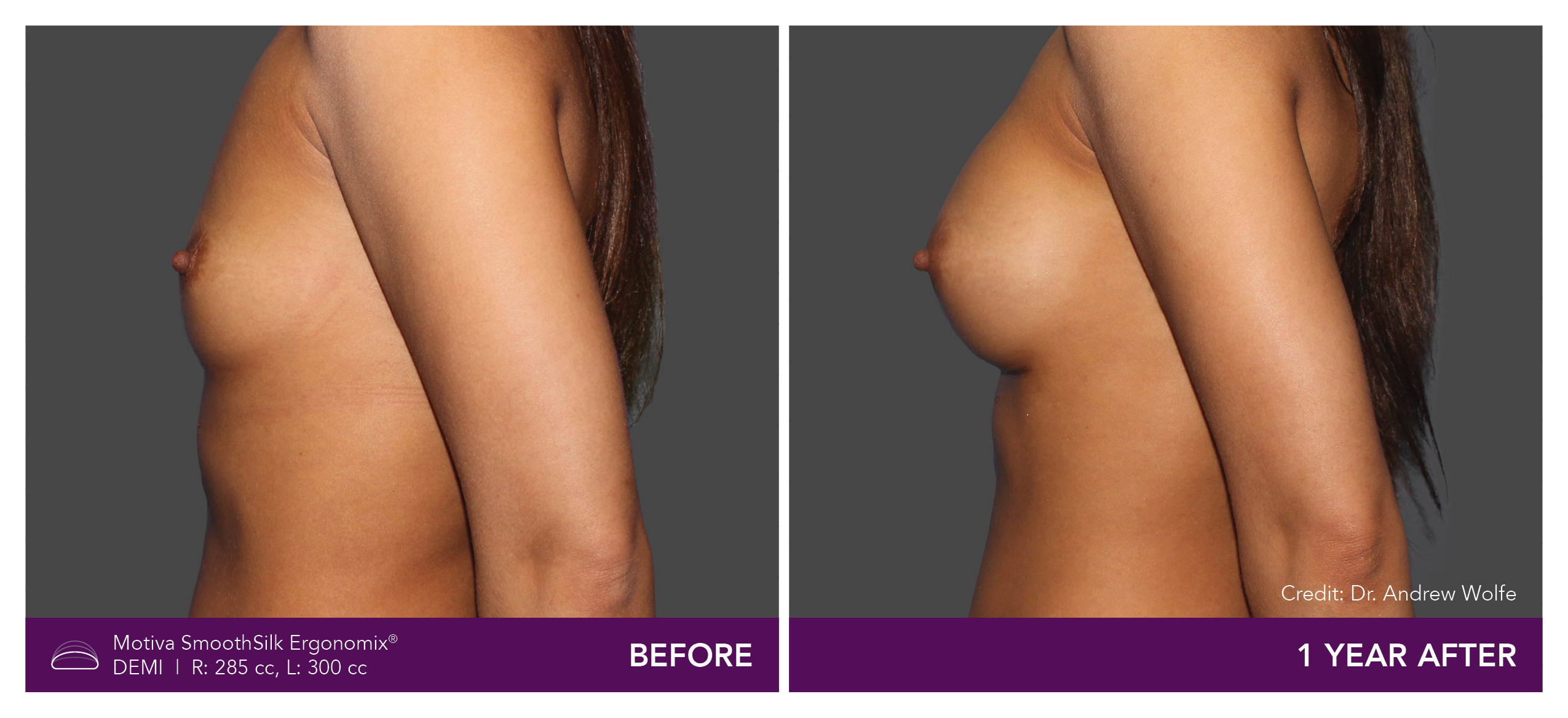
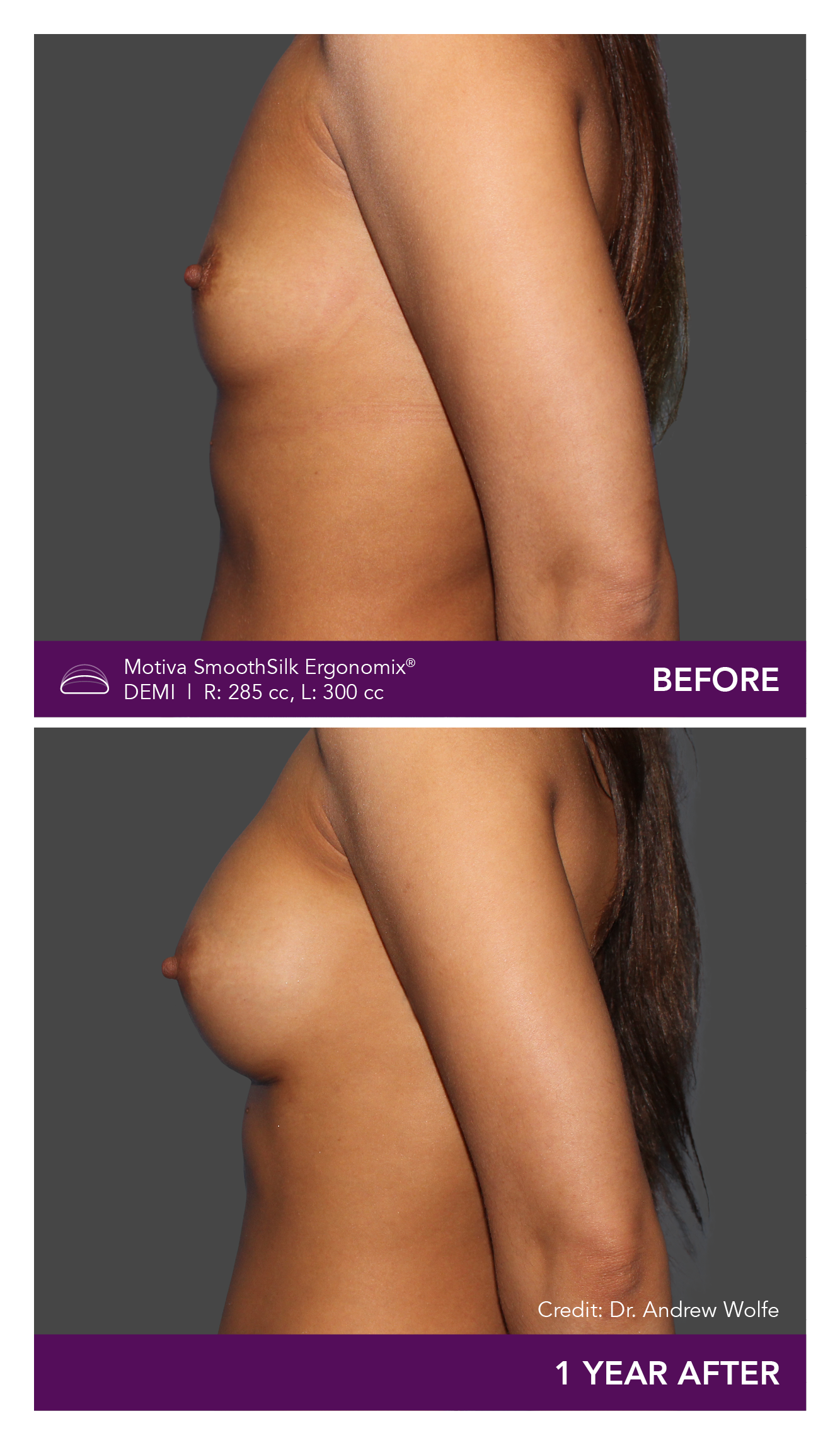
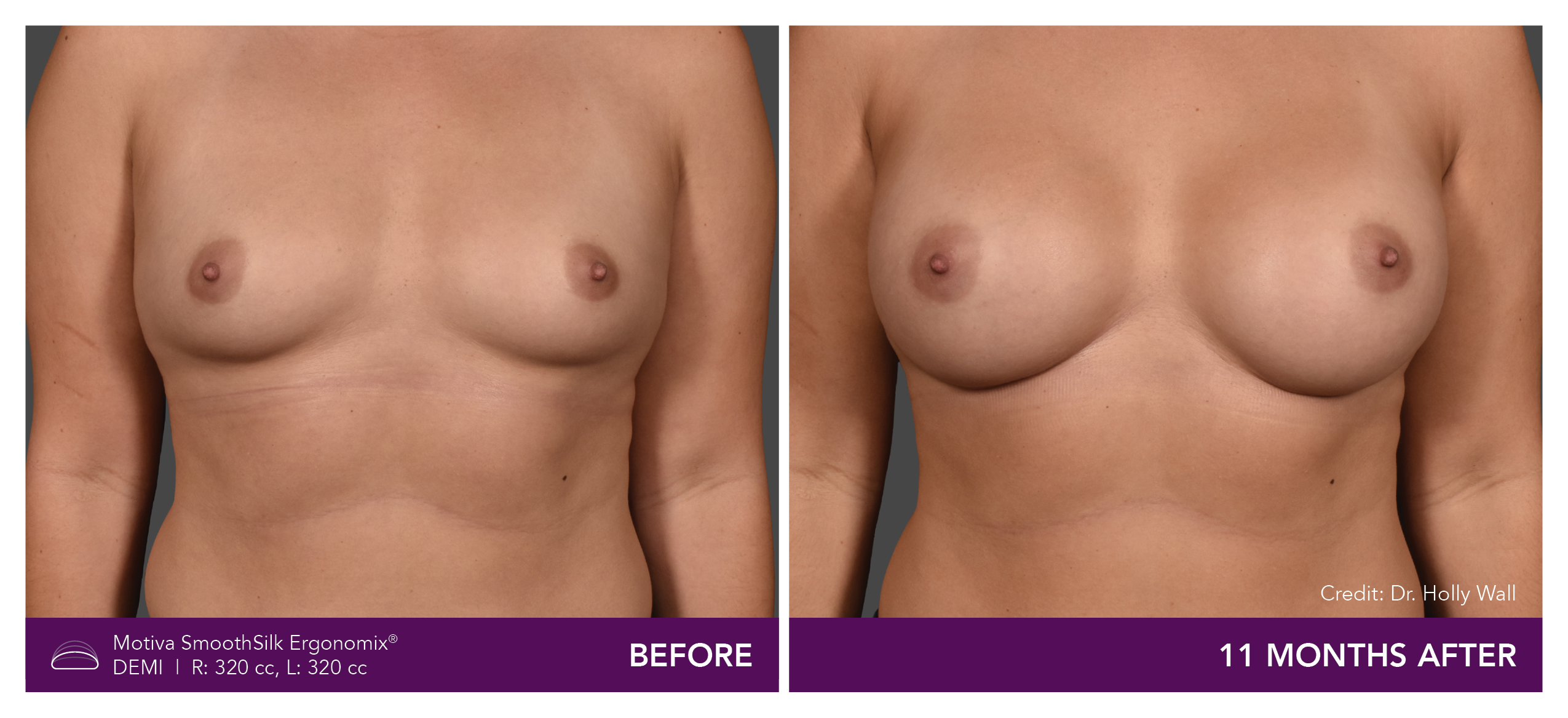
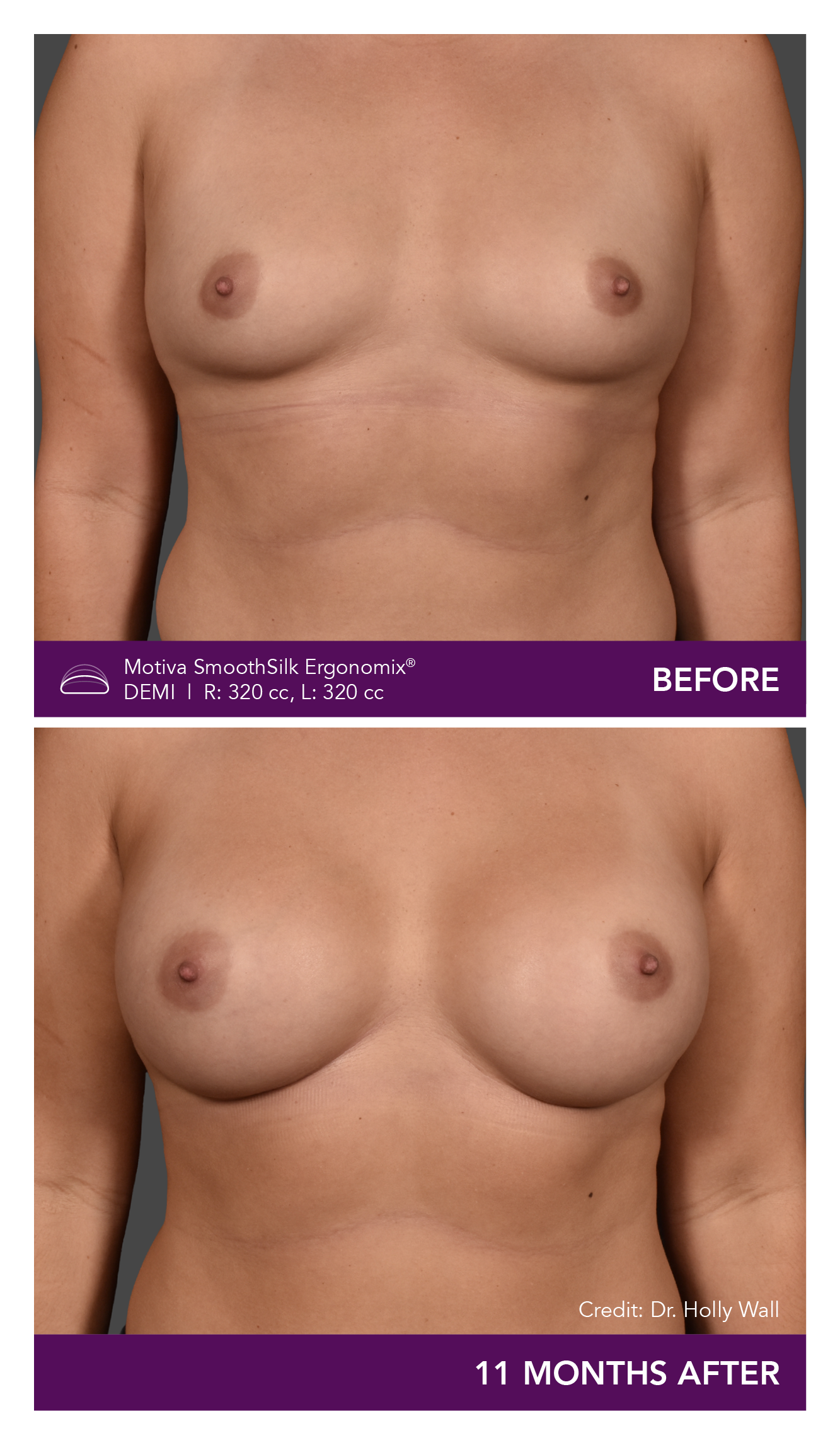
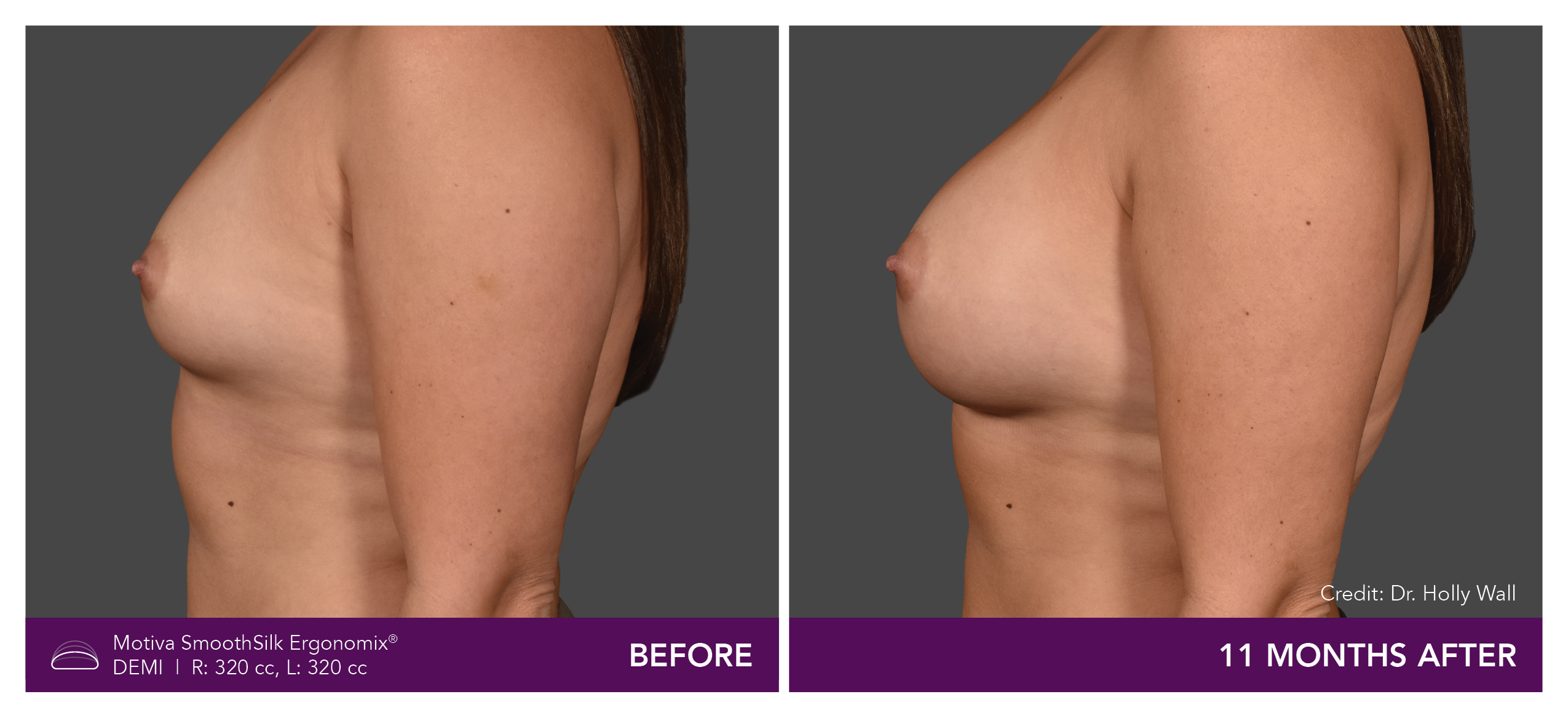
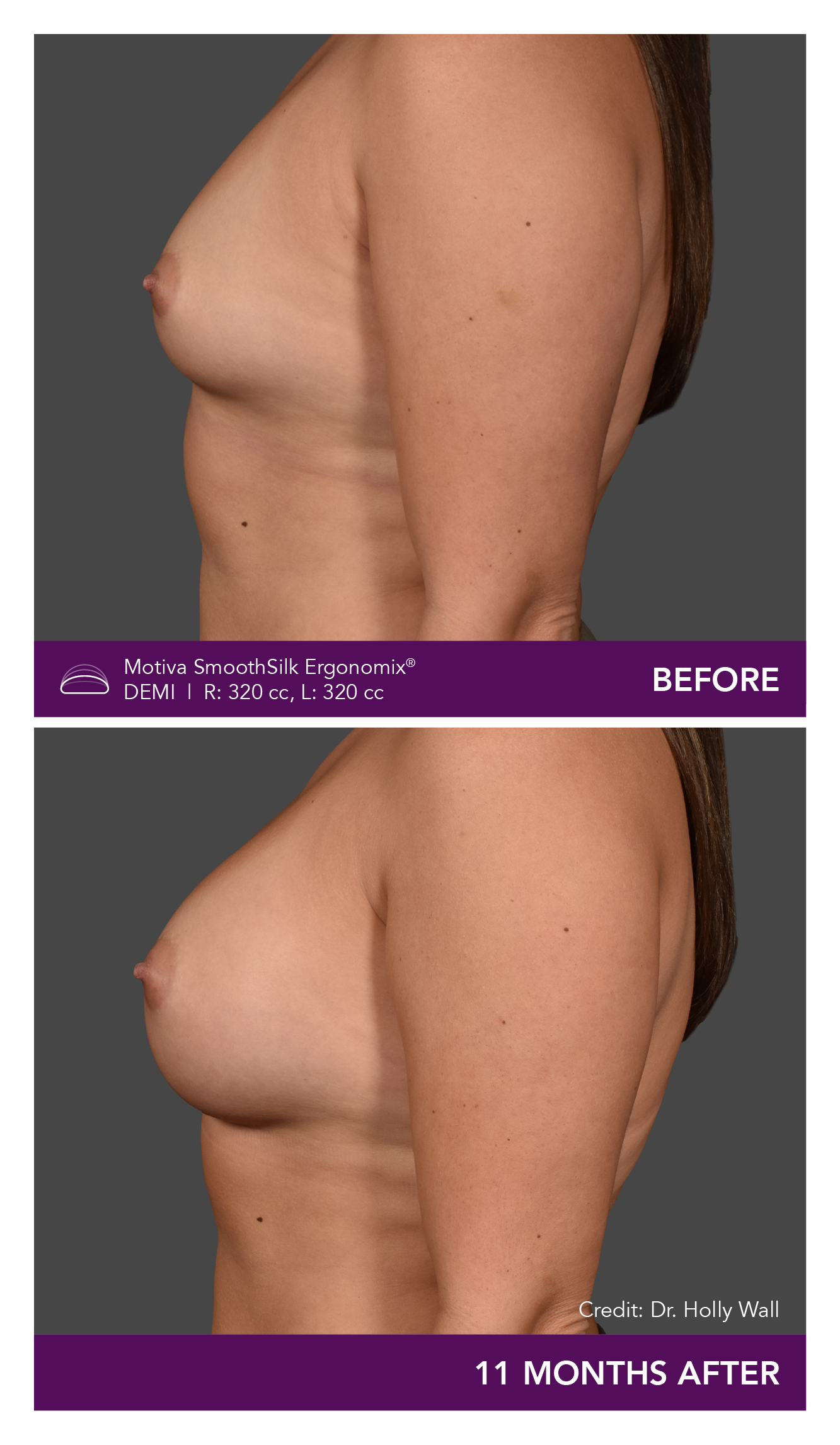
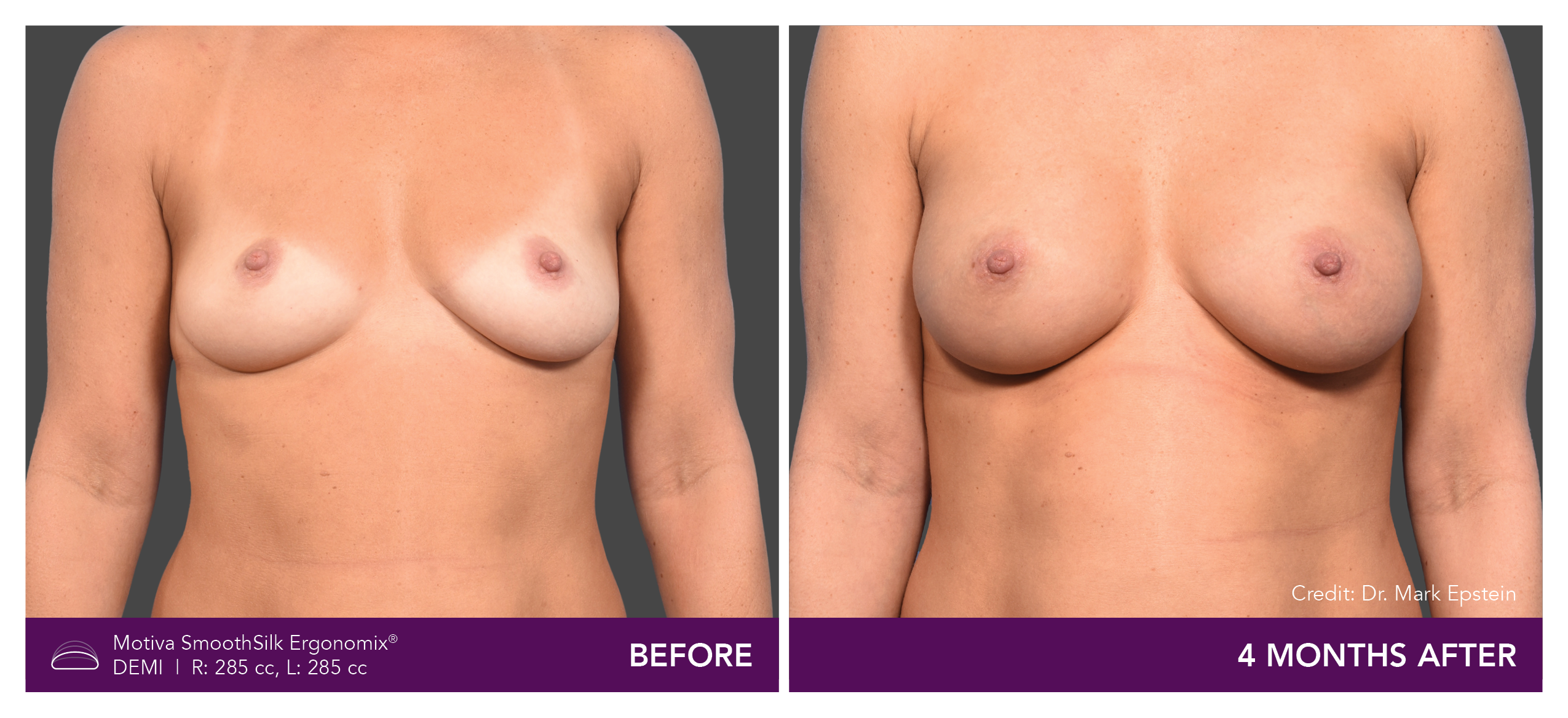
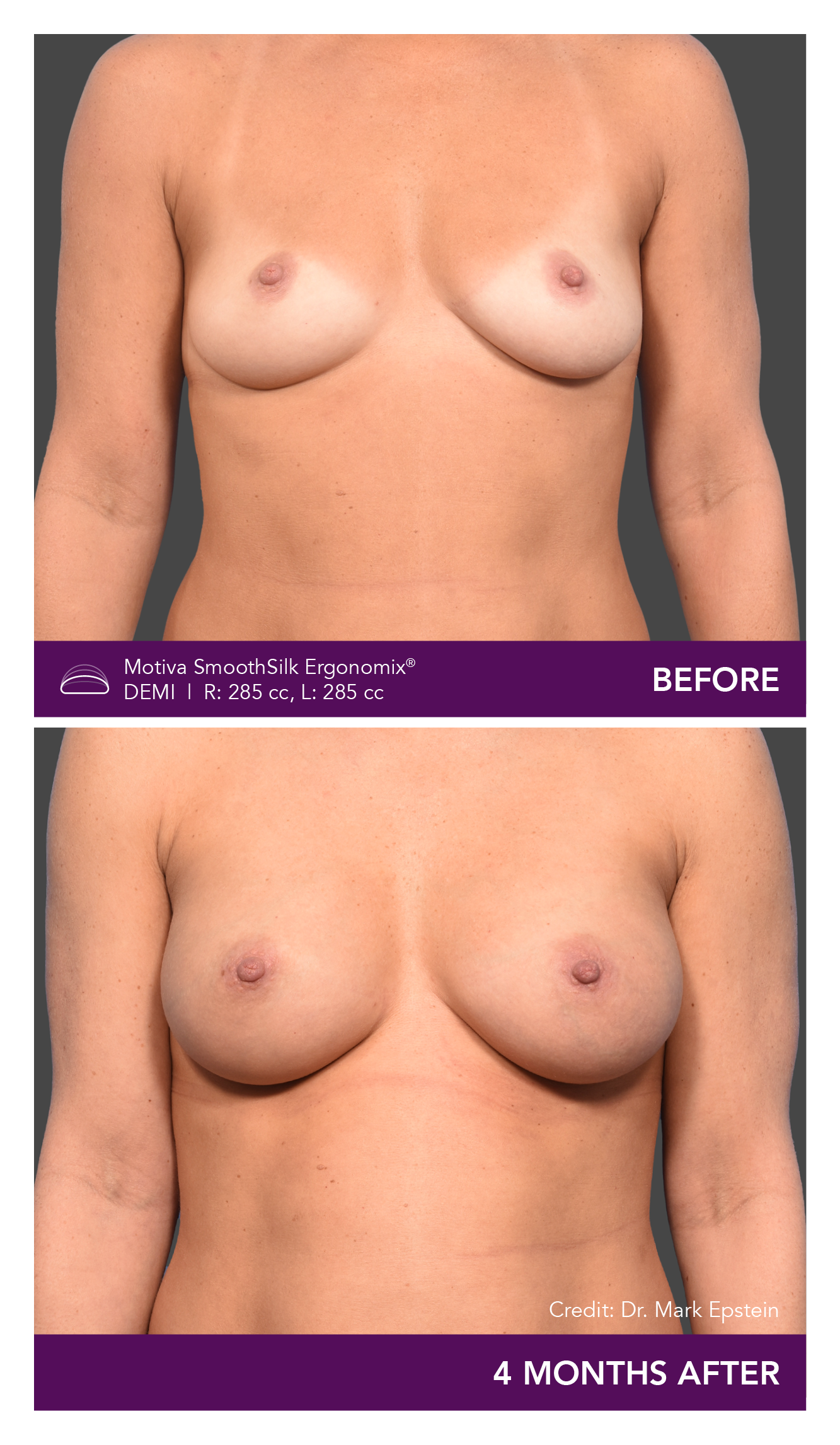
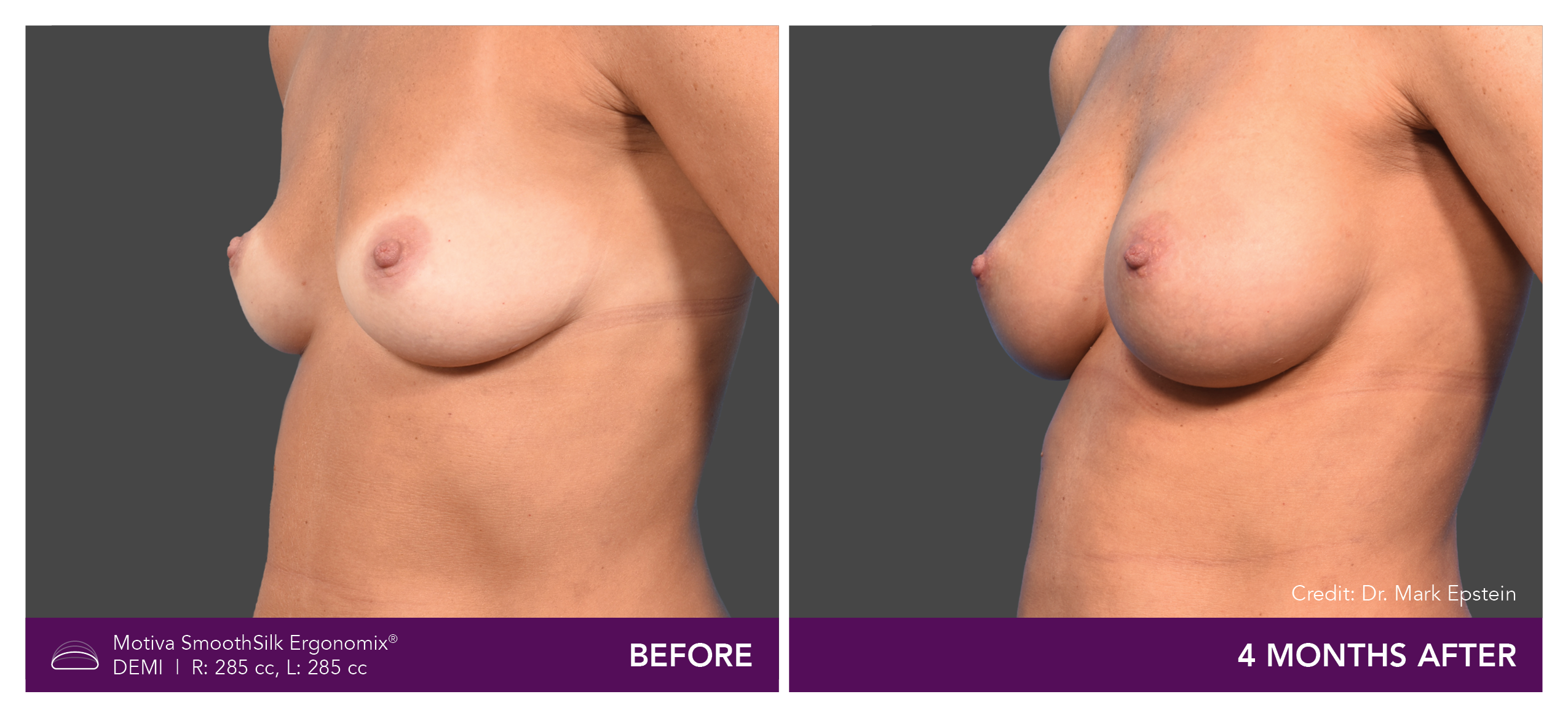
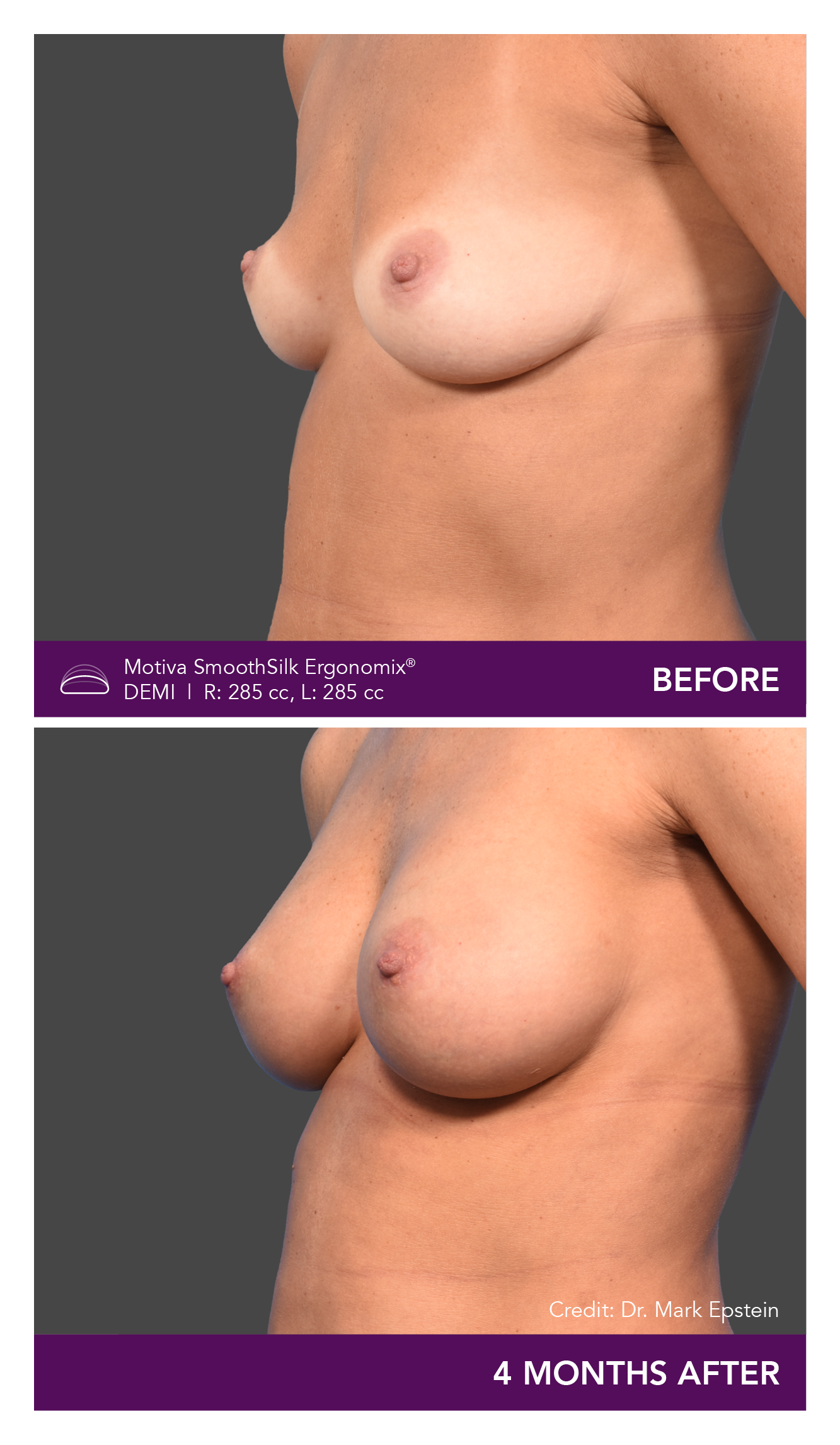
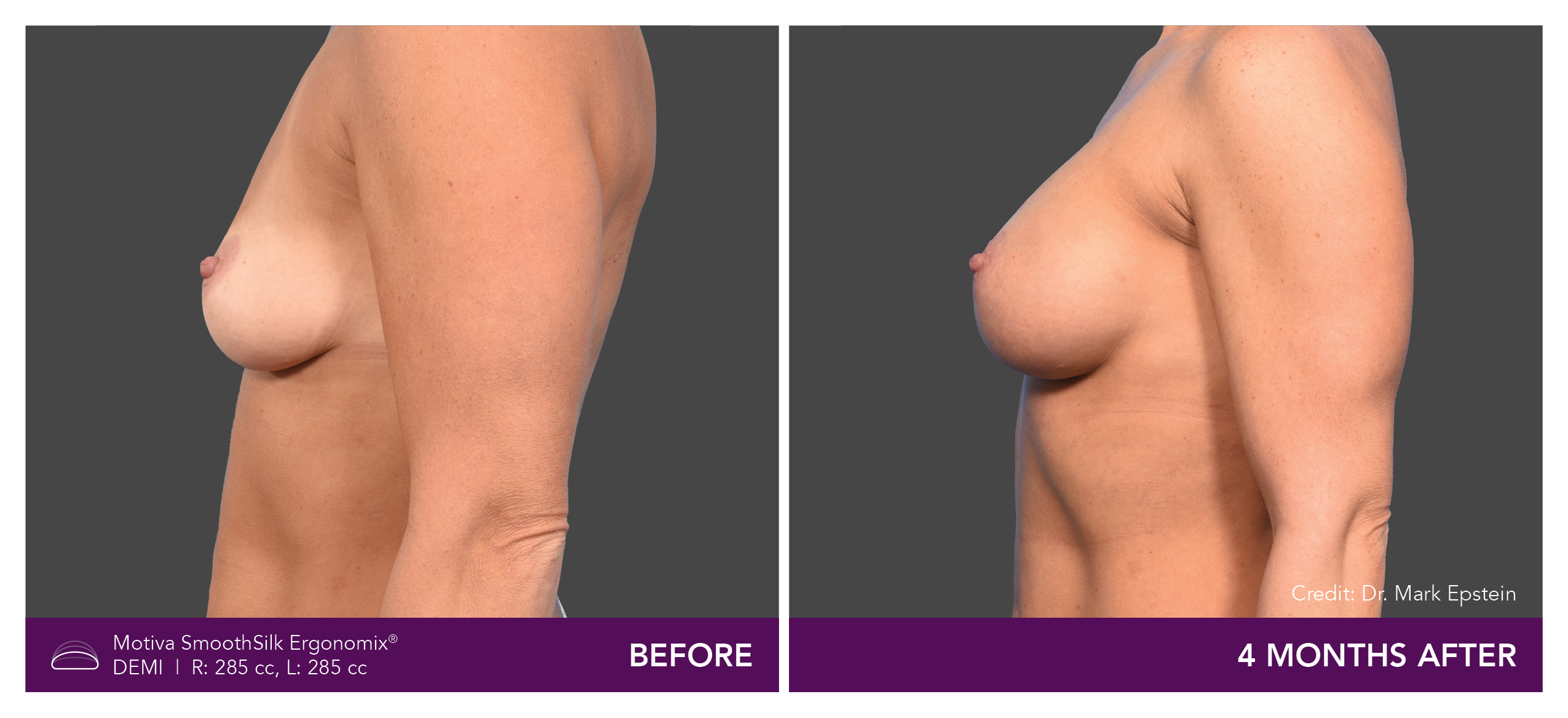
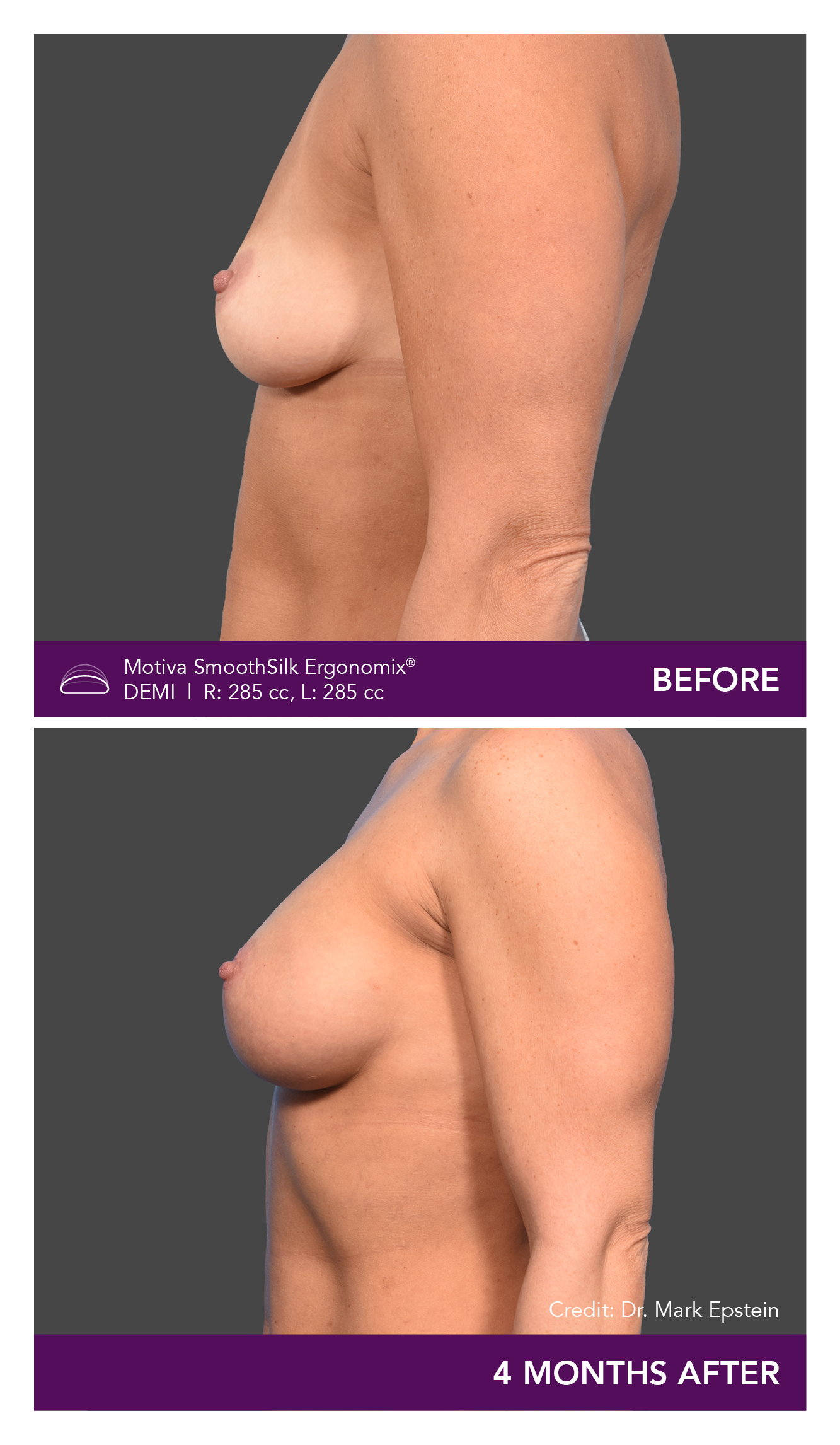
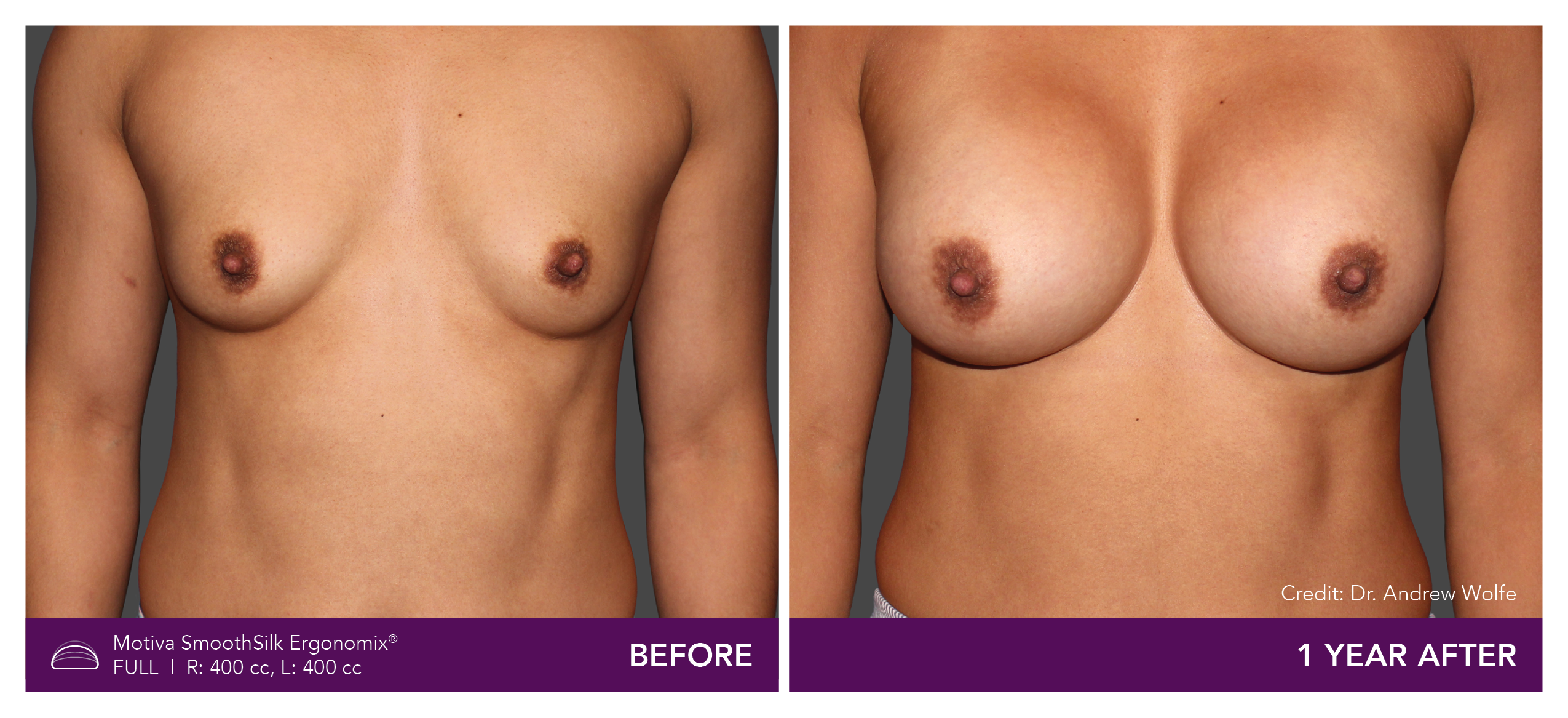
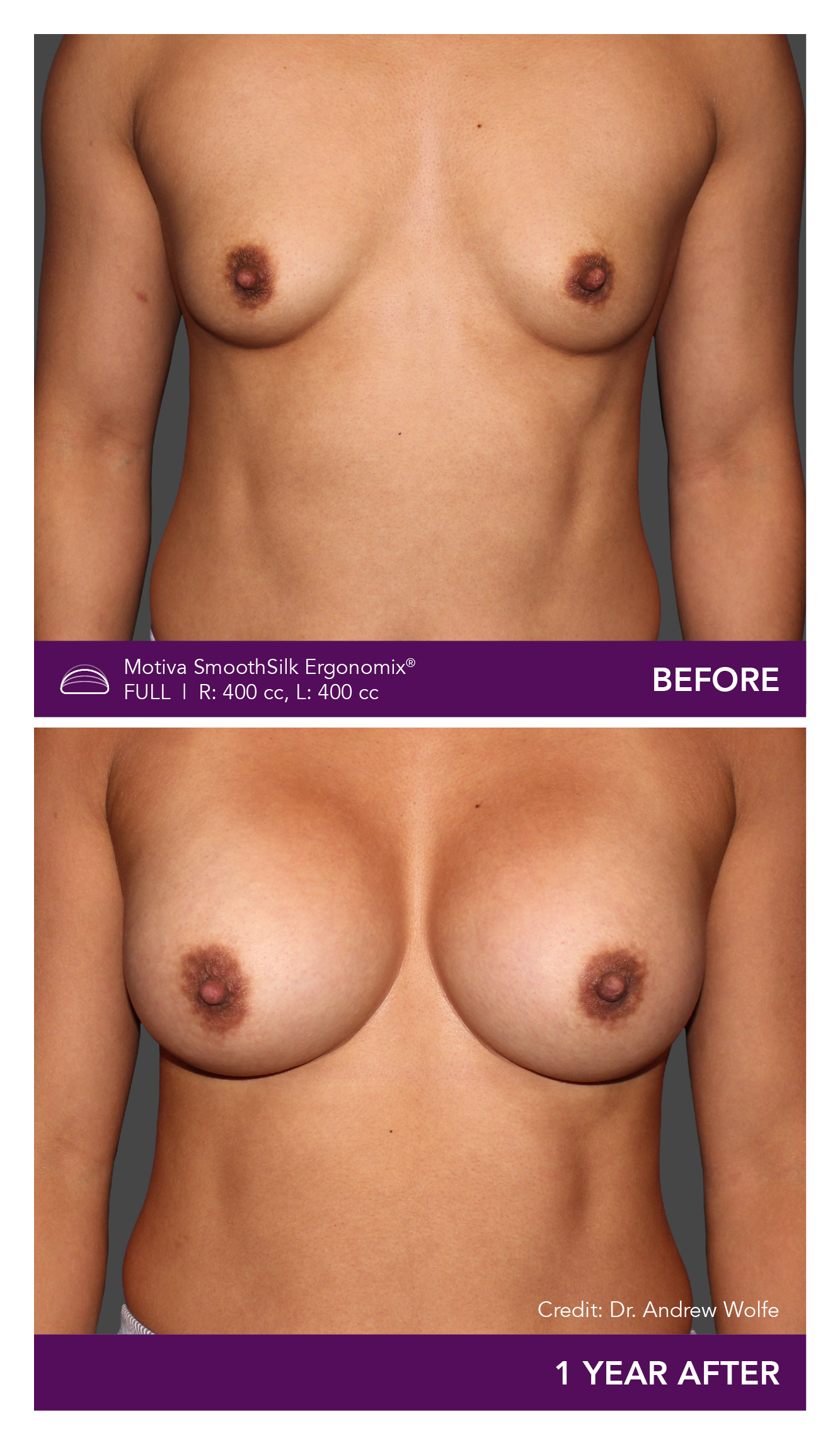
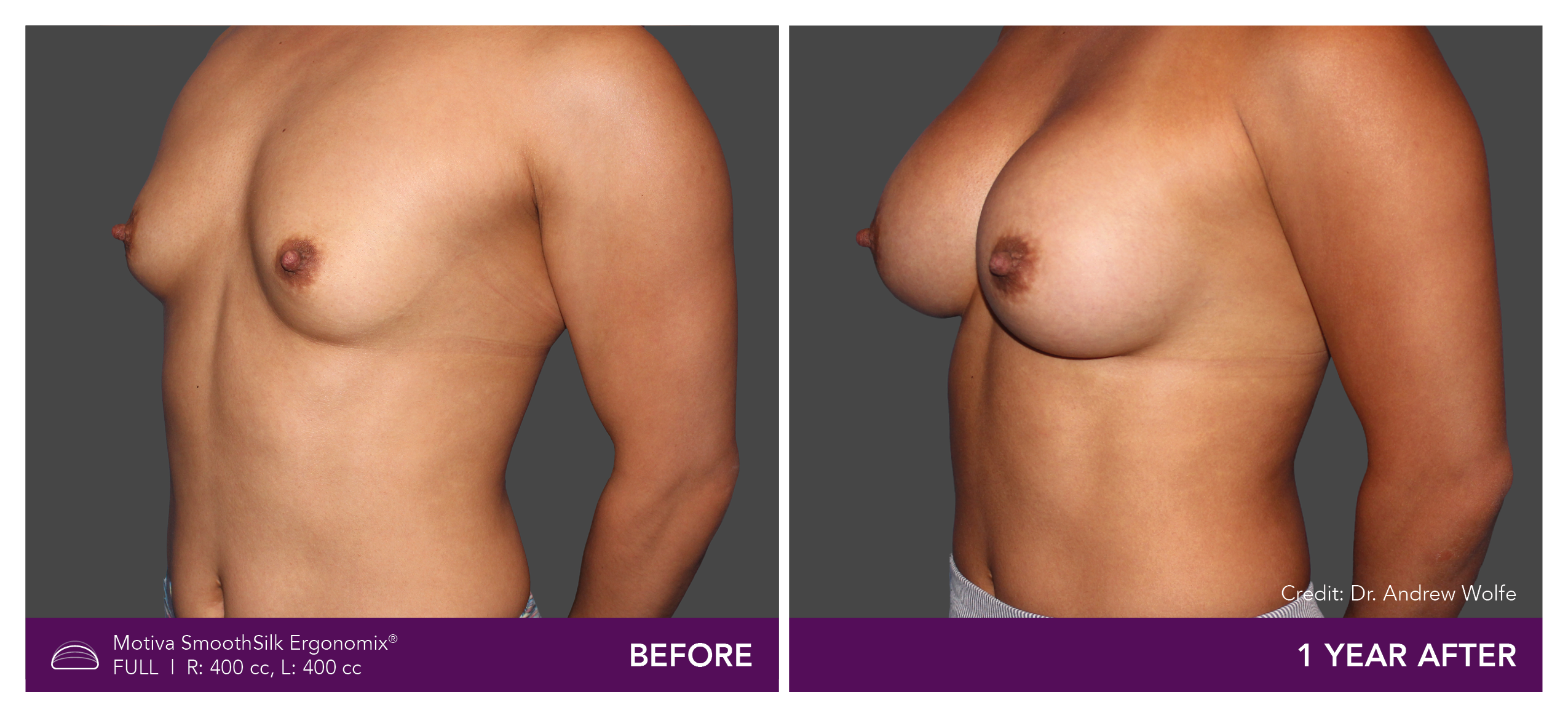
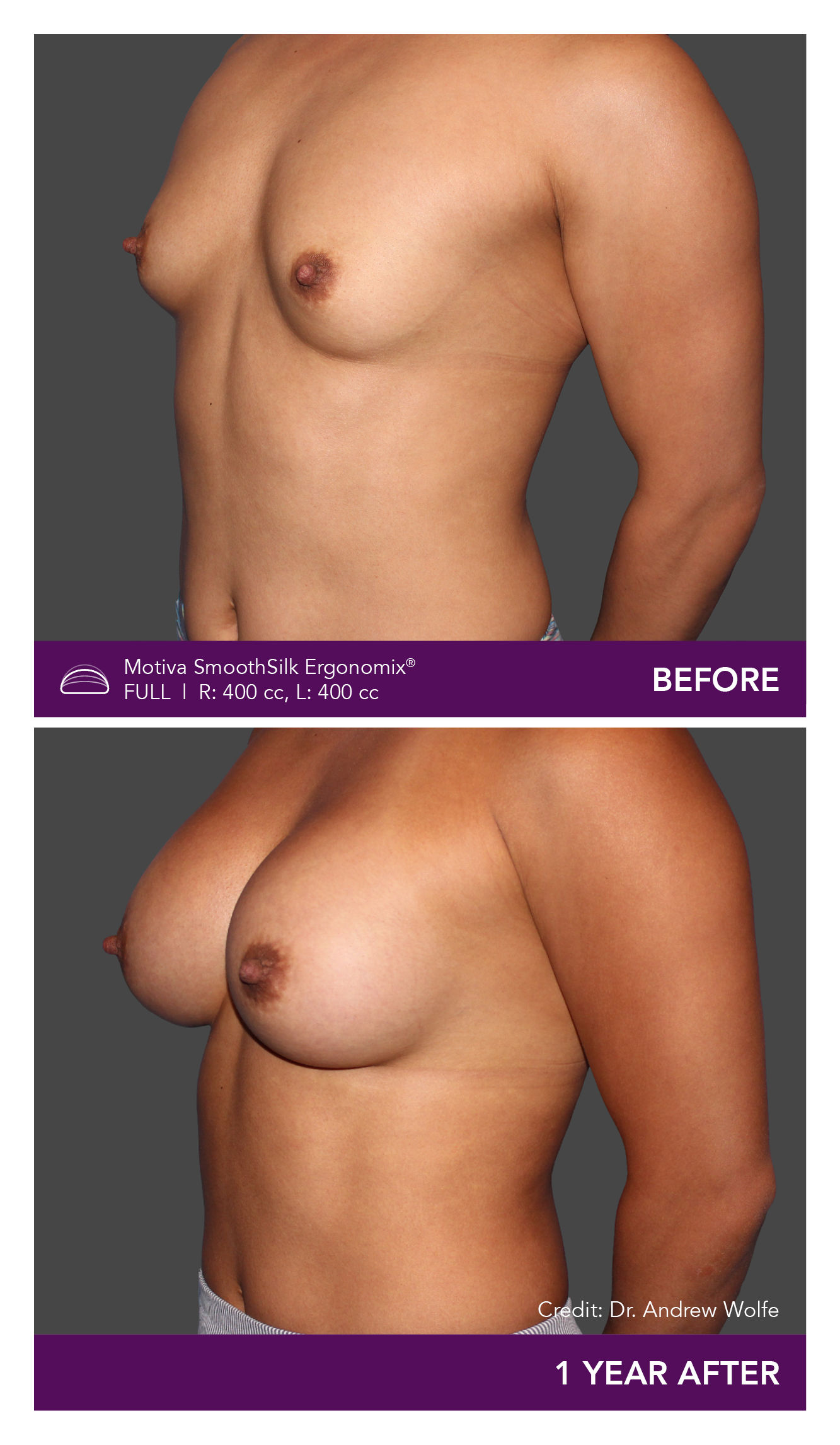
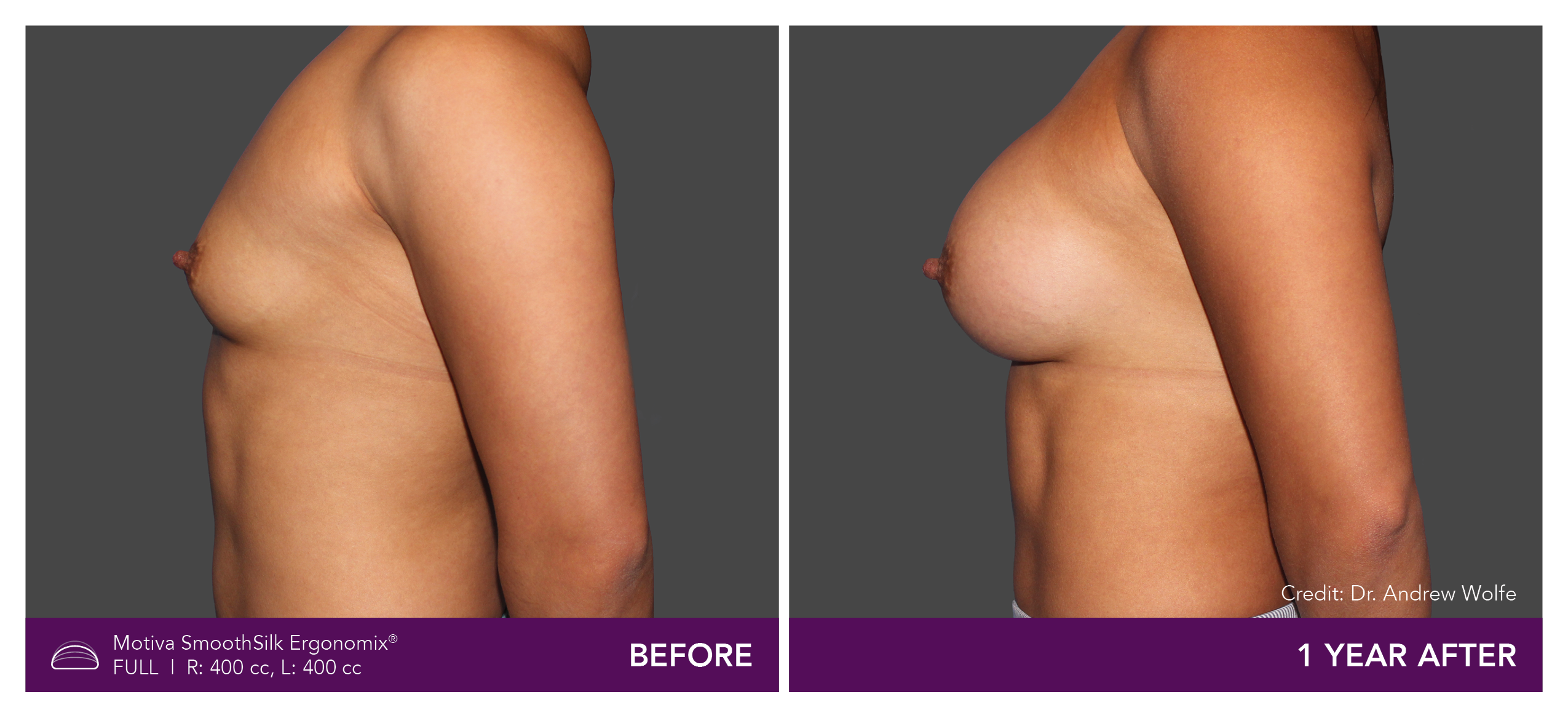
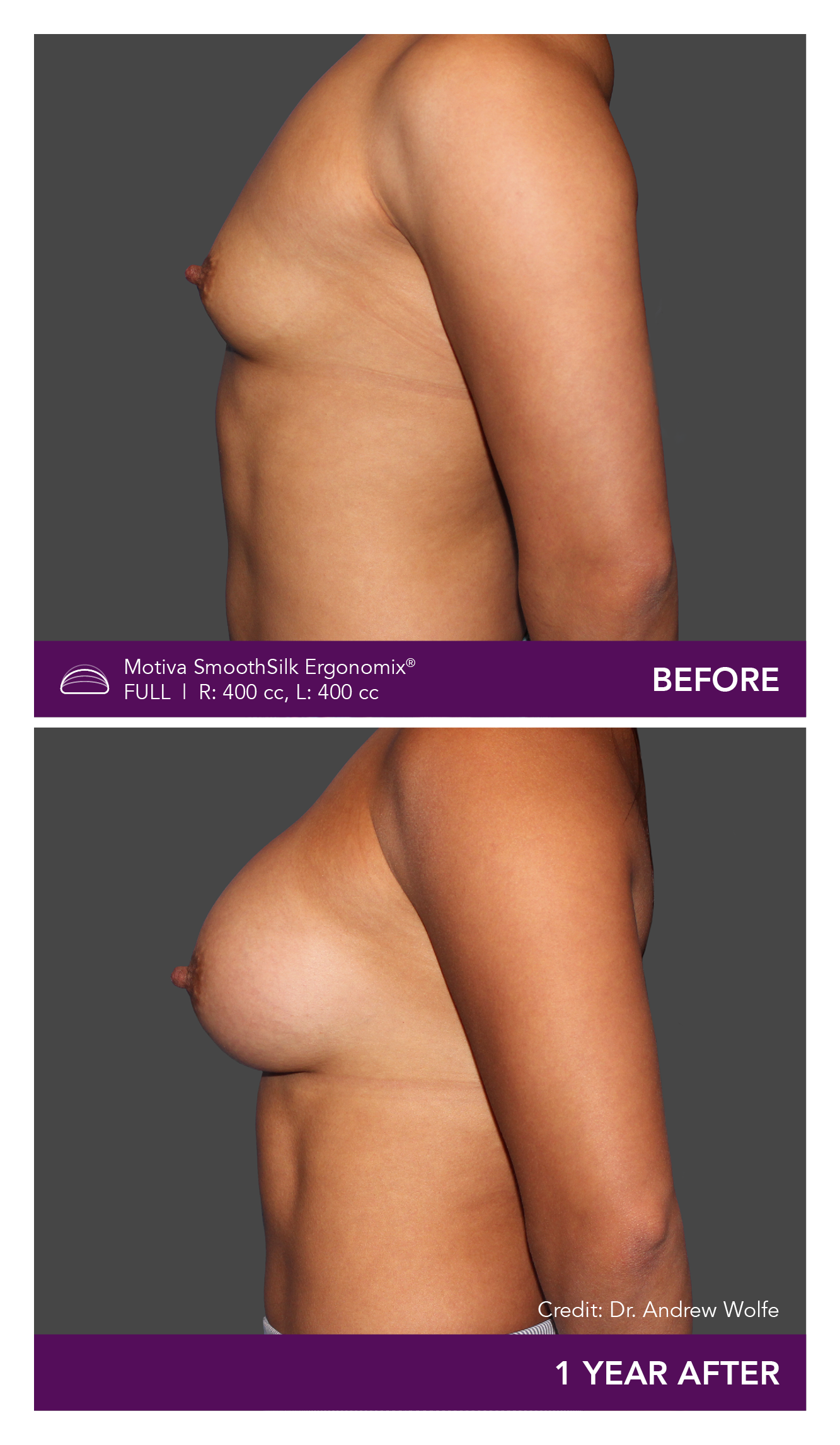
Aesthetic Breast Augmentation
Motiva Implants® showcase cutting-edge innovation in plastic surgery,
offering surgeons and patients advanced Femtech solutions for breast aesthetics.
Focused on women’s health and well-being, Motiva® takes pride in partnering
with board-certified plastic surgeons by making their practice and patients' safety1,12 our primary motivation.
Motivated by next-level innovation
All Motiva Implants® feature:
-

Viscoelastic ProgressiveGel®
100% filled implants consisting of a 6th generation viscoelastic and highly cohesive silicone-gel.2,3,4
-

TrueMonobloc® Shell
The next-generation gel-bonding technology that unifies the shell, patch, and gel into one single unit.5
-

SmoothSilk®
SmoothSilk® is a 4-micron surface,6 designed for enhanced biocompatibility and scientifically shown to promote a low inflammatory response,6 low friction,7 and low bacterial attachment.8,9
-

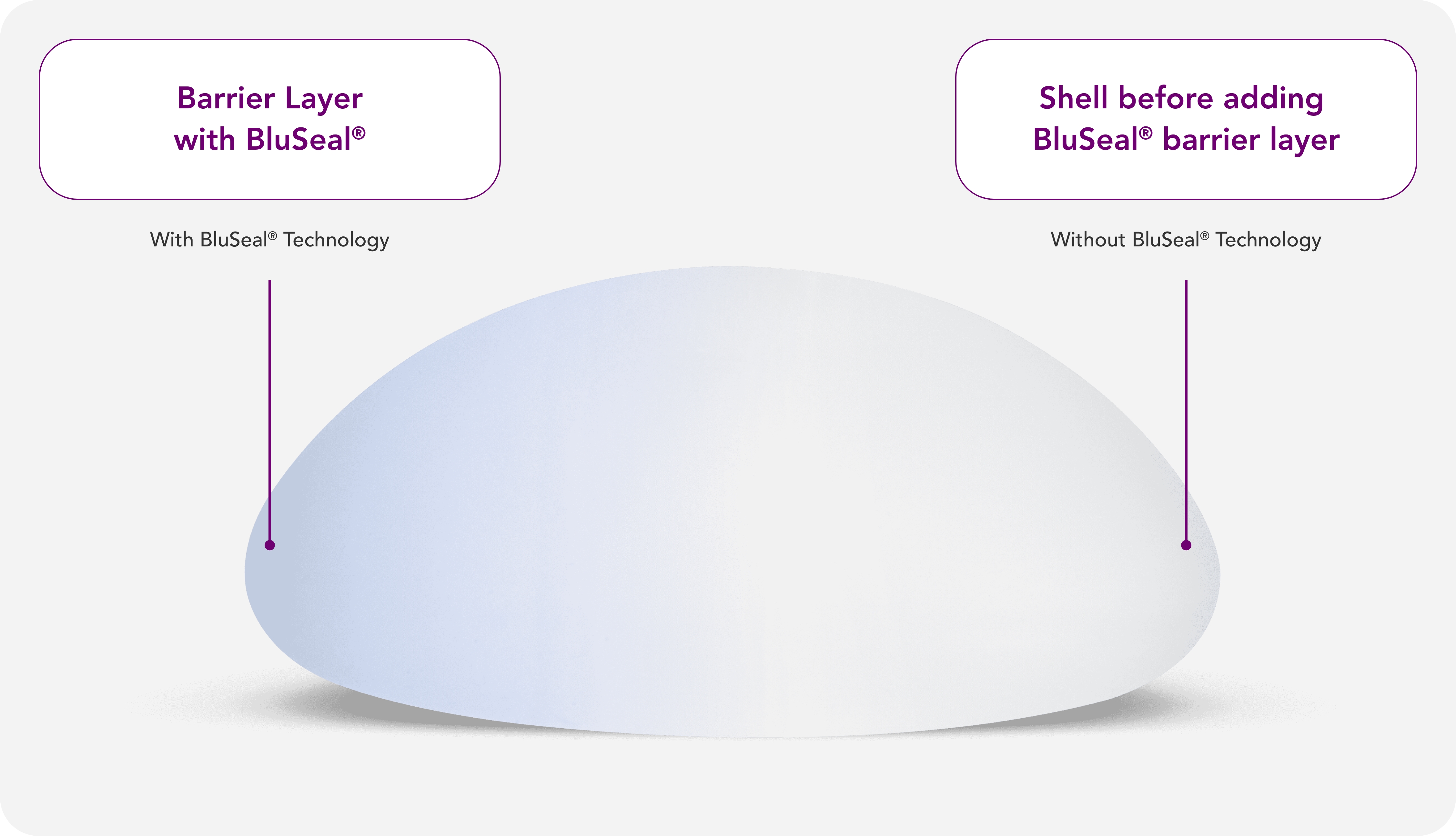
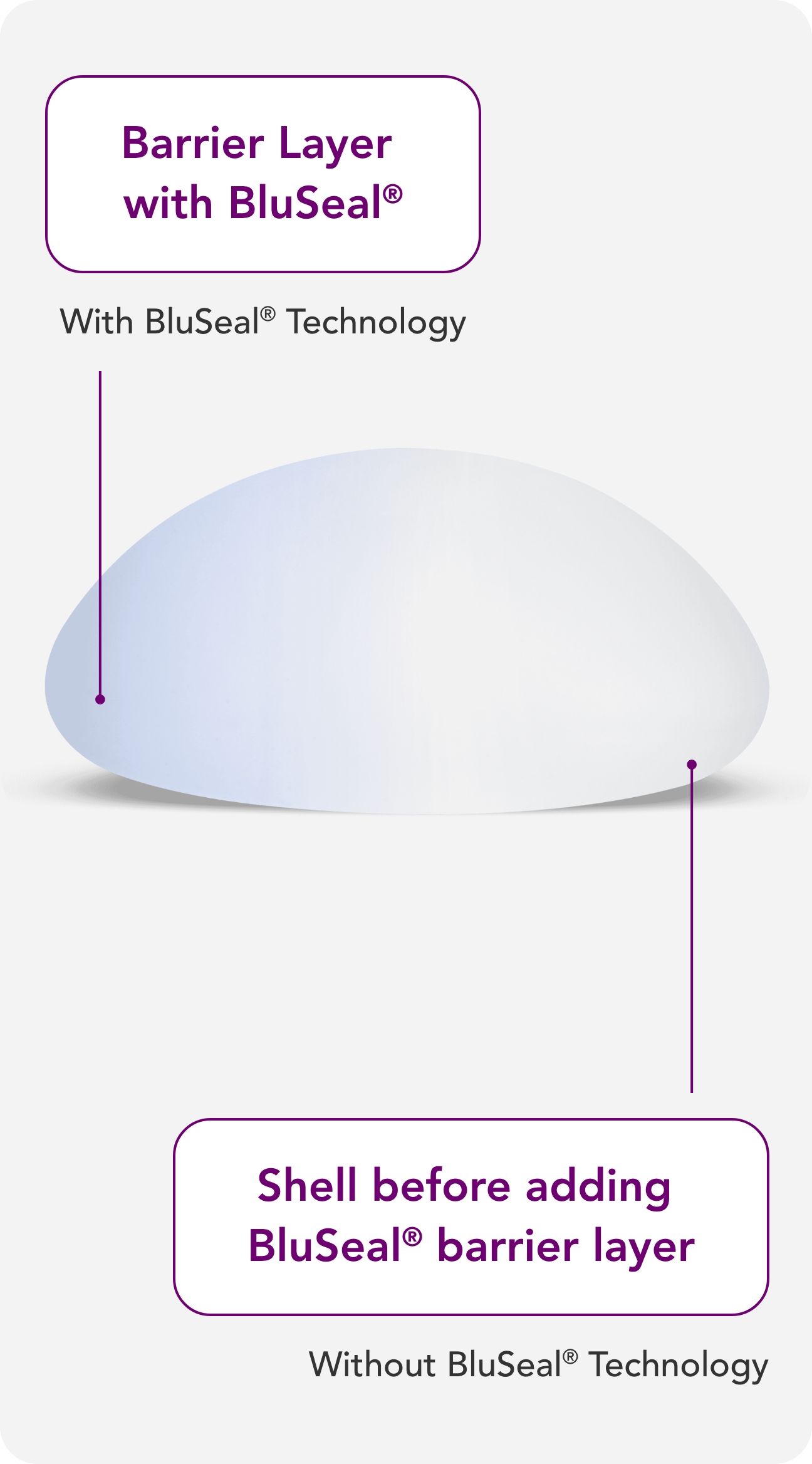
BluSeal®
A visual indicator of the barrier layer that confirms the integrity of the external and internal components of the implant, for quality assurance and patient safety.2,3
*As compared to SmoothSilk® Round Implant.
Motivated by breakthrough
science that starts at the surface
SmoothSilk® is a revolutionary biocompatible* 4-micron surface6 produced by the mandrel imprinting technique11 and designed to optimize the body’s immune response.
This proprietary surface is scientifically shown to promote:
- low inflammatory response,6
- low friction,7
- low bacterial attachment,8,9
resulting in soft breasts, low capsular contracture rates,1,12 and no reported primary device-related proliferative diseases (BIA-ALCL, BIA-SCC and B-cell lymphomas).13
*According to the International Standard ISO 10993-1:2018 and to the Saline, Silicone Gel, and Alternative Breast Implants Guidance for Industry and Food and Drug Administration Staff, September 29, 2020.

Motivated by unmatched gel shell integration
A shell for every gel
TrueMonobloc® is the next-generation gel-bonding technology that unifies the shell, patch, and gel into one single unit.5
Each Motiva® Implant is specifically designed with a unique shell that perfectly matches each gel and allows for maximum gel shell integration, adaptability, and dynamics.
Motivated by uncompromised softness, shape and dynamics
Motiva Implants® are 100% filled with the latest, 6th generation gel that is both highly cohesive and viscoelastic.
This gel composition is designed to result in14:
• Less folds
• Less friction
• Lower risk of rupture


Motivated by safety12,15 you can see
Barrier layers are crucial in breast implant shells, as they may help reduce the risk of patient exposure to low-weight molecular siloxanes.15
With BluSeal®, Motiva® provides a visual indicator of the barrier layer that confirms the integrity of the external and internal components of the implant, designed for quality assurance and patient safety.12,15
Motivated by unmatched safety and satisfaction
97.1% of primary augmentation patients, and 99.0% of the physicians were satisfied with their results.*16
Motiva® 4-year Key Complication Rates
(Kaplan-Meier, KM), including MRI cohort
| Primary augmentation |
2-year (N=451) 95%CI17 |
3-year (N=451) 95%Cl18 |
4-year (N=451) 95%Cl1 |
|---|---|---|---|
|
Capsular contracture (Baker Grade III/IV) |
0.5% | 0.5% | 0.5% |
|
Rupture, suspected or confirmed (MRI Cohort)1 |
0.6% | 0.6% | 0.6% |
|
Breast pain |
0.5% | 0.7% | 0.9% |
|
Infection |
0.9% | 0.9% | 0.9% |
|
Implant removal, with or without replacement |
1.6% | 1.6% | 1.8% |
|
Any reoperation2 |
5.7% | 6.1% | 6.8% |
|
Any complication3 |
7.5% | 8.4% | 9.6% |
All data presented is preliminary 2, 3, and 4-year follow up IDE Study data and does not reflect the final study results nor establish the ultimate safety or effectiveness of the device.
*Based on the 5-point Likert Scale. The office-visit follow-up rate at 3-years follow-up was of 92.7%.
References
1. MRI cohort, N=176
2. Any surgery on the breast or chest area, device or non-device related, including size change
3. Any device or non-device related event, including reoperation


Motivated by a commitment to women’s health
It’s not just a warranty, it’s our commitment to women’s health
Coverage with Confidence
|
Motiva® Health Program1* |
Motiva® Health Program Plus1** |
Allergan Confidence Plus® 2 |
MENTOR Promise™ 3 |
MENTOR Promise™ Enhanced 3 |
Sientra Platinum 20™ 4 |
|
|---|---|---|---|---|---|---|
|
Surgery Date As Of |
9/26/2024 |
9/26/2024 |
5/1/2020 |
1/1/2021 |
1/1/2021 |
5/1/2018 |
|
Cost |
Free |
$250 for Round and Ergonomix® |
Free |
Free |
$300 |
Free |
Rupture |
||||||
|
Implant Replacement |
Lifetime |
Lifetime |
Lifetime |
Lifetime |
Lifetime |
Lifetime |
|
Contralateral Implant Replacement |
|
|
|
|
|
|
|
Financial Assistance |
$3,500 – 10 YR |
$5,000 – 10 YR |
$3,500 – 10 YR |
$3,500 – 10 YR |
$3,500 – 10 YR |
$5,000 – 20 YR |
Capsular Contracture (Baker Grade III or IV) |
||||||
|
Implant Replacement |
10 Years |
10 Years |
10 Years |
10 Years |
10 Years |
20 Years |
|
Contralateral Implant Replacement |
|
|
|
|
|
|
|
Financial Assistance |
$2,000 – 2 YR |
$2,500 – 5 YR |
$2,000 – 2 YR |
$2,000 – 2 YR |
$3,500 – 10 YR |
$2,000 – 2 YR |
|
Primary Augmentation |
|
|
|
|
|
|
|
Revision Augmentation |
|
$2,500 2 YR |
|
|
|
|
Late Forming Seroma |
||||||
|
Implant Replacement |
10 Years |
Lifetime |
20 Years |
10 Years |
10 Years |
20 Years |
|
Contralateral Implant Replacement |
|
|
|
|
|
|
|
Financial Assistance |
$0 |
$2,000 – 2 YR |
|
$0 |
$3,500 – 10 YR |
$2,000 – 2 YR |
|
Primary Augmentation |
|
|
|
|
|
|
|
Revision Augmentation |
|
|
|
|
|
|
Double Capsule |
||||||
|
Implant Replacement |
10 Years |
Lifetime |
|
10 Years |
10 Years |
20 Years |
|
Contralateral Implant Replacement |
|
|
|
|
|
|
|
Financial Assistance |
$0 |
$3,500 – 2 YR |
|
$0 |
$3,500 – 10 YR |
$2,000 – 2 YR |
|
Primary Augmentation |
|
|
|
|
|
|
|
Revision Augmentation |
|
|
|
|
|
|
BIA-ALCL |
||||||
|
CD30 Panel Testing/ Late Seroma |
|
|
$1,000 |
|
|
|
|
Implant Replacement |
Lifetime |
Lifetime |
|
|
|
20 Years |
|
Financial Assistance5 |
$7,500 |
Up to $15,000 – 10 YR*** |
$7,500 |
$7,500 |
$7,500 |
$7,500 – 20 YR |
|
Contralateral Replacement |
|
|
|
|
|
|
Femtech Freedom**** |
||||||
|
Implant Explant |
$0 |
$2,500 - 2 YR |
$0 |
$0 |
$0 |
$0 |
* It is recommended that all patients should register within 90 days of surgery. Subject to full program Terms & Conditions
** Registration and payment will be required to be eligible for the Motiva® Health Program Plus. Subject to full program Terms & Conditions
*** Coverage will be $7,500 after 10 years
**** Subject to full program Terms & Conditions
References
1. Motiva® USA LLC. Motiva® Health Program and Motiva® Health Program Plus Warranty Program. 2024
2. Allergan, Inc. The natrelle confidenceplus warranty program. Allergan; May 2020.
3. Mentor Worldwide LLC. Mentorpromise and mentorpromise enhanced protection plan for memorygel breast implants and memoryshape breast implants terms and conditions. Mentor Corpororation; 2021.
4. Sientra, Inc. Sientra Platinum20 warranty program. Sientra; May 2018.
5. For financial assistance of uninsured patients, a see the BIA-ALCL Patient Assistance Fund Application at https://www.aserf.org/images/documents/bia-alcl-patient-fund-grant-request-form.pdf.
References
1. Establishment Labs Holdings. Establishment Labs Notes Presentation of 4-Year Results from Motiva U.S. IDE Study at The Aesthetic Meeting 2024. https:// investors.establishmentlabs.com/press-releases/pressreleasesdetails/2024/Establishment-Labs-NotesPresentation-of-4-Year-Results-from-Motiva-U.S.- IDEStudy-at-The-Aesthetic-Meeting-2024/default.aspx. Published May 2nd, 2024. Accessed May 28th, 2024.
2. Establishment Labs, DDD-001: Device Description Document Sterile Silicone Breast Implants Motiva Implants® Round SmoothSilk®/SilkSurface® Plus. Data on File.
3. Establishment Labs, DDD-002: Device Description Document for Sterile Silicone Breast Implants Motiva Implants® Ergonomix® Round SmoothSilk®/SilkSurface®. Data on File.
4. Establishment Labs, TR-001038: Rheological analysis of silicone filling gels of Motiva Implants® and other brands’ silicone filling gels using the BTC-2000. Data on File.
5. Establishment Labs, TS-001196: Mechanical and peel testing study of Motiva Implants® to support the TrueMonobloc® technology. Data on File.
6. Doloff JC, Veiseh O, Mezerville R de, et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nature Biomedical Engineering 2021. Published online June 21, 2021:1-16. doi:10.1038/s41551-021-00739-4
7. Rosas JM, Atkins DJ, Chau AL, et al. In vitro models of soft tissue damage by implant associated frictional shear stresses. Proceedings of the Institution of Mechanical Engineers, Part J: Journal of Engineering Tribology. 2022;0(0). doi:10.1177/13506501221132897
8. James G, Boegli L, Hancock J et al. Bacterial Adhesion and Biofilm Formation on Textured Breast Implant Shell Materials. Aesth Plast Surg. 2019 Apr; 43:490–497. doi: 10.1007/s00266-018-1234-7
9. Jones P, Mempin M, Hu H, et al. The functional influence of breast implant outer shell morphology on bacterial attachment and growth. Plast Reconstr Surg. 2018;142(4):837- 849. doi: 10.1097/PRS.0000000000004801
10. Zeplin PH. Narbensparende Brustvergrößerung: Erfahrungen mit über 500 Implantaten. Handchirurgie · Mikrochirurgie · Plastische Chirurgie. 2021;53(02):144-148. doi:10.1055/a-1307-3917
11. Barr S, Hill EW, Bayat A. Functional biocompatibility testing of silicone breast implants and a novel classification system based on surface roughness.J Mech Behav Biomed Mater. 2017. Nov;75:75- 81. doi:10.1016/j.jmbbm.2017.06.030
12. Aitzetmuller-Kleitz ML, Yang S, Wiebinghaus P, Wellenbrock S, Ozturk M, Kuckelhaus M et al. Complication rates after breast surgery with the Motiva Smooth SilkSurface silicone gel implants – A systematic review and meta-analysis. Clin. Med. 2023, 12,1881. doi: 10.3390/jcm12051881
13. Establishment Labs®, Post-Market Surveillance Preliminary Results Q1 2024. Internal Data on File.
14. Brandon HJ, Taylor ML, Powell TE, Walker PS. Morphology of Breast Implant Fold Flaw Failure. J Long Term Eff Med Implants. 2006. 16(6):441-450. doi: 10.1615/jlongtermeffmedimplants.v16vi6.40
15. Establishment Labs, TSD-001019: Technical Summary Document Assessment of silicone diffusion/ gel bleed from Motiva Implants ®, according to ISO 14607:2018 method. Data on File.
16. Glicksman C, Wolfe A, McGuire P. The Study of the Safety and Effectiveness of Motiva SmoothSilk Silicone Gel-Filled Breast Implants in Patients Undergoing Primary and Revisional Breast Augmentation: Three-Year Clinical Data. Aesthet Surg J. 2024 Sep 27:sjae134. doi: 10.1093/asj/sjae134. Epub ahead of print. PMID: 39331509.
17. Establishment Labs Holdings Corp. Establishment Labs Notes Presentation of 2-Year Results from Motiva U.S. IDE Study at The Aesthetic Meeting 2022. Establishment Labs Holdings Corp. – Establishment Labs Notes Presentation of 2-Year Results from Motiva U.S. IDE Study at The Aesthetic Meeting 2022. Published April 21th, 2022. Accessed May 28th, 2024.
18. Establishment Labs Holdings Corp. Establishment Labs Notes Presentation of 3-Year Results from Motiva U.S. IDE Study at The Aesthetic Meeting 2023. Establishment Labs Holdings Corp. – Establishment Labs Notes Presentation of 3-Year Results from Motiva U.S. IDE Study at The Aesthetic Meeting 2023. Published April 20th, 2023. Accessed May 28th, 2024.
Disclaimer
The sale and distribution of this device is restricted to users and/or user facilities that provide information to patients about the risks and benefits of this device in the form and manner specified in the approved labeling provided by Establishment Labs® and Motiva® USA. Federal (USA) Law restricts this device to sale by or on the order of a physician.
The Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast Implants are indicated for breast augmentation for women at least 22 years old. Breast augmentation includes primary breast surgery to increase the breast size, as well as revision surgery to correct or improve the result of an original primary breast augmentation surgery (i.e., revision-augmentation). Breast Implant surgery is contraindicated in women with active infection anywhere in their bodies, with existing cancer or pre-cancer of their breast who have not received sufficient treatment for those conditions, or who are currently pregnant or nursing. Prior to use, plastic surgeons should review all risk information with women who are considering breast implant surgery. This risk information can be found in the Directions for Use which is provided with each device and found on www.motivausa.com. Plastic surgeons should advise patients of the key complications that have been historically associated with breast implant surgery and implantation of silicone gel breast implants, including, but not limited to, capsular contracture, implant removal, reoperation, infection, and rupture. Plastic surgeons should also advise patients that breast implants are not lifetime devices and patients should follow-up with them, as recommended. The Directions for Use and detailed information regarding the risks and benefits of Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast implants can be found at: www.motivausa.com or by calling Motiva at 1-800- 924-5072.
Motiva®, Establishment Labs®, Aesthetic BreastRecon®, Motiva Implants®, SmoothSilk®, ProgressiveGel® TrueMonobloc®, BluSeal®, Ergonomix®, ProgressiveGel Plus®, and ProgressiveGel Ultima® are trademarks of Establishment Labs Holdings® Inc.