Motivated by women’s health.
Driven by Femtech.
Motiva® by Establishment Labs® is a first-of-its-kind medical technology company focused on breast aesthetics and breast reconstruction*, bringing Femtech innovations to the market for women’s health and well-being.
*Motiva Implants® are not approved in the United States for reconstruction indication.


14+ years of global innovation1
-
~4M
Devices
Establishment Labs® devices** in worldwide market since 2010.
-
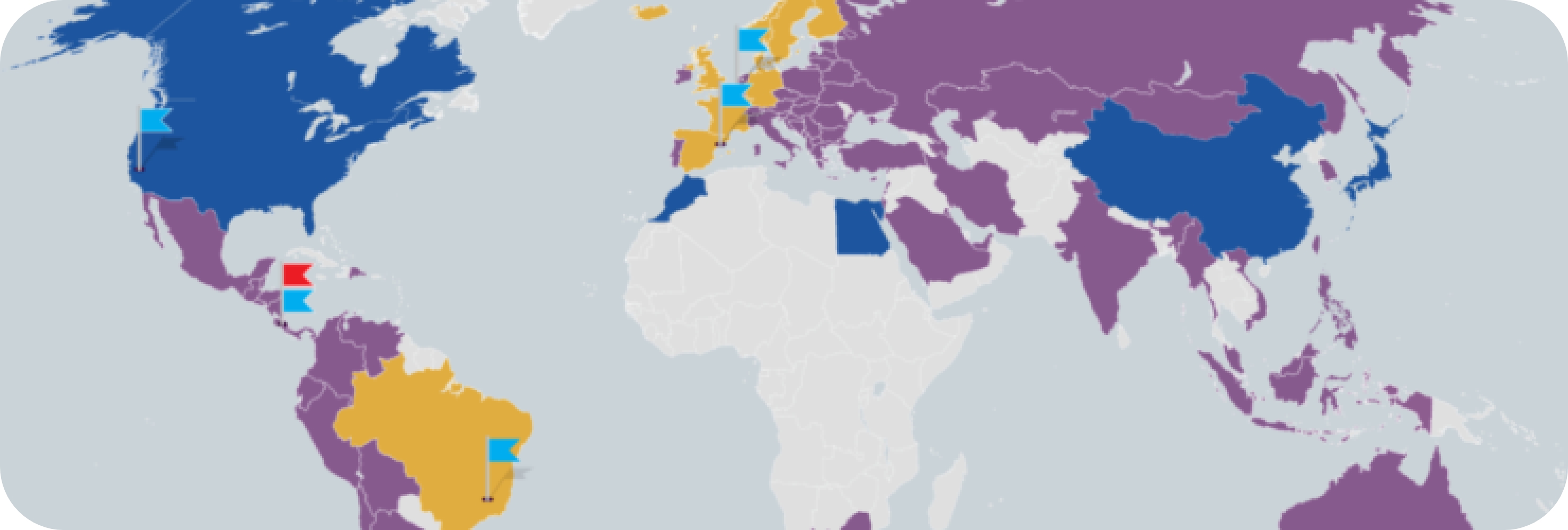
86+
Countries
where Establishment Labs® devices** have been approved.
-
140+
Publications
Establishment Labs® actively supports research.
-
210+
Patents
Applications approved/pending in 21 jurisdictions.
**This includes breast implants and breast tissue expanders worldwide.


Driven by Femtech
More than two decades ago, Motiva® by Establishment Labs® changed the industry paradigm by putting women’s health first. With innovation, safety, and technology, we started a revolution in the Femtech sphere by creating a new space that cared for women’s unmet needs within the breast aesthetic and reconstruction fields. The main goal: to create Femtech solutions that heal, normalize, and democratize breast health and aesthetics.
Our Headquarters
SULÀYÖM INNOVATION CAMPUS
The Heart of Creation is our hub for innovation in Costa Rica, bringing together women’s health contributors, patients, healthcare professionals, and partners. Inspired by the Sulàyöm mountain’s creation tale, our campus includes the Global Learning Center, an amphitheater for medical community growth, an in-house content studio, and Research, Development, and Innovation labs.


Timeline
2004
Company founded, and research started.
2010
Motiva Implants® launch.

2011
CE Mark obtained in Europe.

2018
FDA clinical trial initiated, Establishment Labs® goes public on NASDAQ stock market.

2021
Landmark peer reviewed publication in Nature Biomedical Engineering: Surface topography of silicone breast implants mediates the foreign body response in mice, rabbits, and humans.

2023
Presented 3-year data from FDA clinical trial demonstrating less than 1% device-related complications.2

2023
Opening of our Innovation Campus – Sulàyöm in San José, Costa Rica.

2023
FDA Clearance of Motiva Flora® SmoothSilk® Tissue Expander.

2023
First Motiva Flora® surgery in the United States of America.3

2024
Launch of Motiva® in China. Approved in 86+ countries.1
2024
FDA approval of Motiva Smoothsilk® Round and Smoothsilk® Ergonomix® Silicone Gel Breast Implants for aesthetic indication.

Today
Nearly 4 million implants sold over 14 years in over 86 countries, 210+ patents, 140+ publications.1

References
1. Establishment Labs®, Post-Market Surveillance Preliminary Results Q1 2024. Internal Data on File.
2. Establishment Labs Notes Presentation of 3-Year Results from Motiva U.S. IDE Study at The Aesthetic Meeting 2023
3. Establishment Labs. Jan, 2024 Establishment Labs Announces First U.S. Commercial Procedure With Motiva Flora Tissue Expander. (Establishment Labs® Press Release)
Sale and Distribution
The sale and distribution of this device is restricted to users and/or user facilities that provide information to patients about the risks and benefits of this device in the form and manner specified in the approved labeling provided by Establishment Labs® and Motiva® USA. Federal (USA) Law restricts this device to sale by or on the order of a physician.
Important Safety Information for Patients
The Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast Implants are indicated for breast augmentation for women of at least 22 years old. Breast augmentation includes primary breast surgery to increase the breast size, as well as revision surgery to correct or improve the result of an original primary breast augmentation surgery (i.e., revision-augmentation). Breast Implant surgery is contraindicated in women with active infection anywhere in their bodies, with existing cancer or pre-cancer of their breast who have not received sufficient treatment for those conditions, or who are currently pregnant or nursing. Adequate studies have not been performed to confirm the safety of breast implant surgery in women with these conditions or under these circumstances; therefore, if you have any of the conditions or circumstances listed above, breast augmentation surgery with implants should not be performed at this time. Failure to take into consideration these contraindications may increase the risks involved with breast implant surgery and have the potential to cause harm. Patients should be advised that key complications have historically been associated with silicone gel breast surgery and implantation of silicone gel breast implants including, but not limited to, capsular contracture, implant removal, reoperation, infection, and rupture. Further, breast implants are not lifetime devices and patients should visit their healthcare professional, as recommended. For more detailed information about the benefits and risks of Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast Implants, please visit: www.motivausa.com or call Motiva at 1-800-924-5072.
Physician Safety Statement
The Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast Implants are indicated for breast augmentation for women at least 22 years old. Breast augmentation includes primary breast surgery to increase the breast size, as well as revision surgery to correct or improve the result of an original primary breast augmentation surgery (i.e., revision-augmentation). Breast Implant surgery is contraindicated in women with active infection anywhere in their bodies, with existing cancer or pre-cancer of their breast who have not received sufficient treatment for those conditions, or who are currently pregnant or nursing. Prior to use, plastic surgeons should review all risk information with women who are considering breast implant surgery. This risk information can be found in the Directions for Use which is provided with each device and found on www.motivausa.com. Plastic surgeons should advise patients of the key complications that have been historically associated with breast implant surgery and implantation of silicone gel breast implants, including, but not limited to, capsular contracture, implant removal, reoperation, infection, and rupture. Plastic surgeonsshould also advise patients that breast implants are not lifetime devices and patients should follow-up with them, as recommended. The Directions for Use and detailed information regarding the risks and benefits of Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast implants can be found at: www.motivausa.com or by calling Motiva at 1-800-924-5072.
Motiva®, Establishment Labs®, Motiva Implants®, Motiva Flora®, SmoothSilk®, and Ergonomix® are trademarks of Establishment Labs Holdings® Inc.