

Meet Motiva®
Motivated by you
More than two decades ago, Motiva® by Establishment Labs® changed the industry by putting women’s health first. Motiva® is here to support you with next-generation implants at the forefront of safety1 and innovation that are motivated by you.
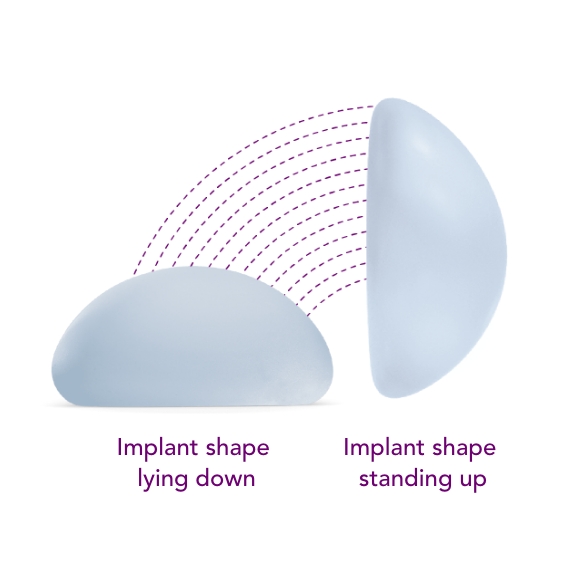
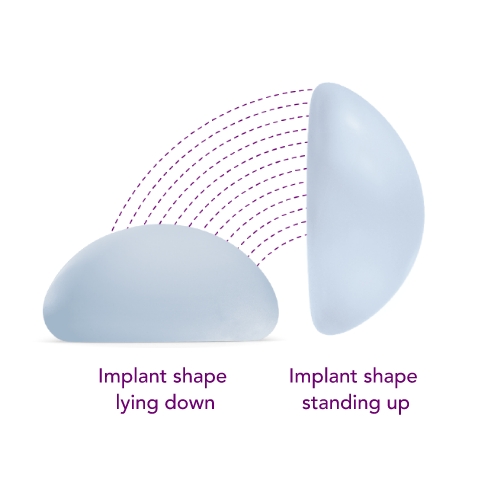
Motiva SmoothSilk® Round
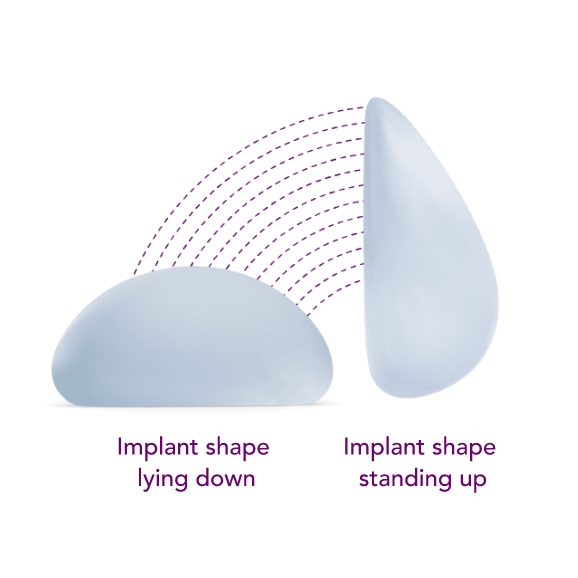
Combines the SmoothSilk® Surface with ProgressiveGel PLUS®, creating increased upper pole fullness and softness.2,3,4 The shape keeps its round, full form regardless of whether you’re standing up or lying down.
-
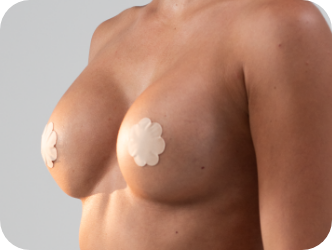
Standing
-
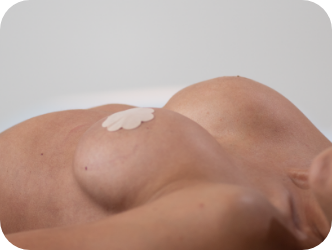
Lying down
-
Benefits of Motiva SmoothSilk® Round
-
Placement
Maintains the highest projection and fullness of the upper breast when standing.2,3,4
-
Look
Perkier and more youthful appearance.2,3,4
-

Feel
Stable soft feel.2,3,4
Motiva SmoothSilk Ergonomix®
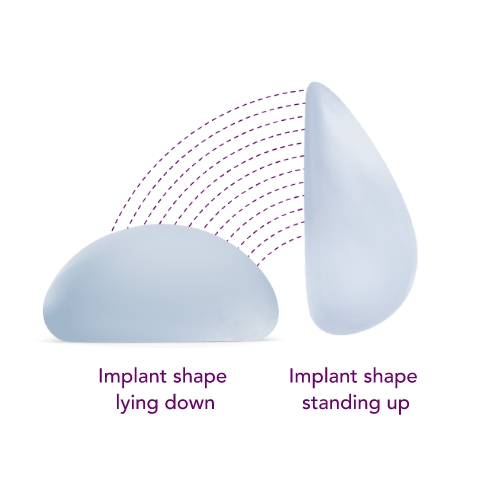
Ergonomix® implants are unique to the implant market, as they can adapt shape as your body changes positions, showcasing a round shape when lying down and a teardrop shape when standing up.3,4,5 This breakthrough implant is designed to be inserted through a small incision6 and minimize scarring.
-

Standing
-
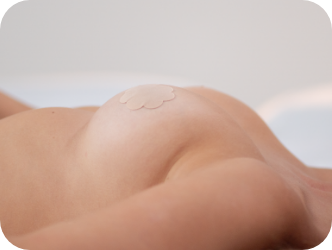
Lying down
-
Benefits of Motiva SmoothSilk Ergonomix®
-

Placement
Motiva SmoothSilk Ergonomix® implants are designed to follow a woman’s movements.3,4,5
-

Look
They hold a round shape when lying down and forms a natural-looking, sloped silhouette when standing.4,5
-

Feel
Motiva SmoothSilk Ergonomix® is able to mimic how real breast tissue looks, feels and moves.3,4,5
References
1. Establishment Labs® Holdings. Establishment Labs® Notes Presentation of 4-Year Results from Motiva® U.S. IDE Study at The Aesthetic Meeting 2024. https://investors.establishmentlabs.com/press-releases/pressreleasesdet…. Published May 2nd, 2024. Accessed May 28th, 2024.
2. Establishment Labs, DDD-001: Device Description Document Sterile Silicone Breast Implants Motiva Implants® Round SmoothSilk® / SilkSurface® Plus. Data on File.
3. Aitzetmuller-Kleitz ML, Yang S, Wiebinghaus P, Wellenbrock S, Ozturk M, Kuckelhaus M et al. Complication rates after breast surgery with the Motiva Smooth SilkSurface silicone gel implants – A systematic review and meta-analysis. Clin. Med. 2023, 12,1881. doi: 10.3390/jcm12051881
4. Establishment Labs, TR-001038: Rheological analysis of silicone filling gels of Motiva Implants® and other brands’ silicone filling gels using the BTC-2000. Data on File.
5. Establishment Labs, DDD-002: Device Description Document for Sterile Silicone Breast Implants Motiva Implants® Ergonomix® Round SmoothSilk®/SilkSurface®. Data on File.
6. Zeplin PH. Narbensparende Brustvergrößerung: Erfahrungen mit über 500 Implantaten. Handchirurgie · Mikrochirurgie · Plastische Chirurgie. 2021;53(02):144-148. doi:10.1055/a-1307-3917
Disclaimer
The sale and distribution of this device is restricted to users and/or user facilities that provide information to patients about the risks and benefits of this device in the form and manner specified in the approved labeling provided by Establishment Labs® and Motiva® USA. Federal (USA) Law restricts this device to sale by or on the order of a physician.
The Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast Implants are indicated for breast augmentation for women at least 22 years old. Breast augmentation includes primary breast surgery to increase the breast size, as well as revision surgery to correct or improve the result of an original primary breast augmentation surgery (i.e., revision-augmentation). Breast Implant surgery is contraindicated in women with active infection anywhere in their bodies, with existing cancer or pre-cancer of their breast who have not received sufficient treatment for those conditions, or who are currently pregnant or nursing. Adequate studies have not been performed to confirm the safety of breast implant surgery in women with these conditions or under these circumstances; therefore, if you have any of the conditions or circumstances listed above, breast augmentation surgery with implants should not be performed at this time. Failure to take into consideration these contraindications may increase the risks involved with breast implant surgery and have the potential to cause harm. Patients should be advised that key complications have historically been associated with silicone gel breast surgery and implantation of silicone gel breast implants including, but not limited to, capsular contracture, implant removal, reoperation, infection, and rupture. Further, breast implants are not lifetime devices and patients should visit their healthcare professional, as recommended. For more detailed information about the benefits and risks of Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast Implants, please visit: www.motivausa.com or call Motiva at 1-800-924-5072.
Motiva®, Establishment Labs®, Aesthetic Breastrecon®, SmoothSilk®, Progressive Gel PLUS®, Ergonomix®, and ProgressiveGel ULTIMA® are trademarks of Establishment Labs Holdings® Inc.