

Next Level Innovation & Safety
Motivated by
the SmoothSilk® Difference
Only Motiva Implants® have SmoothSilk®, a unique surface designed for enhanced biocompatibility, to improve the body’s response to your implants.1 Compared to other implants*, the SmoothSilk® surface was scientifically shown to have the least amount of inflammation1 and low bacterial attachment2,3, helping the tissue around the implant to stay soft.1
*Surfaces studied included the following competitors’ implants: Mentor Smooth Round, Mentor MemoryGel, Allergan Microcell and Allergan Biocell.
Benefits of SmoothSilk®
-
Soft breasts.4,5
-
Low capsular contracture rates4,5 (when the scar tissue surrounding the implant tightens or hardens).
-

No reported primary device-related incidence of BIA-ALCL.6
Motivated by next-level design
Every person is unique, and Motiva® is committed to providing options designed to fit your aesthetic goals.
Motivated by safety you can see
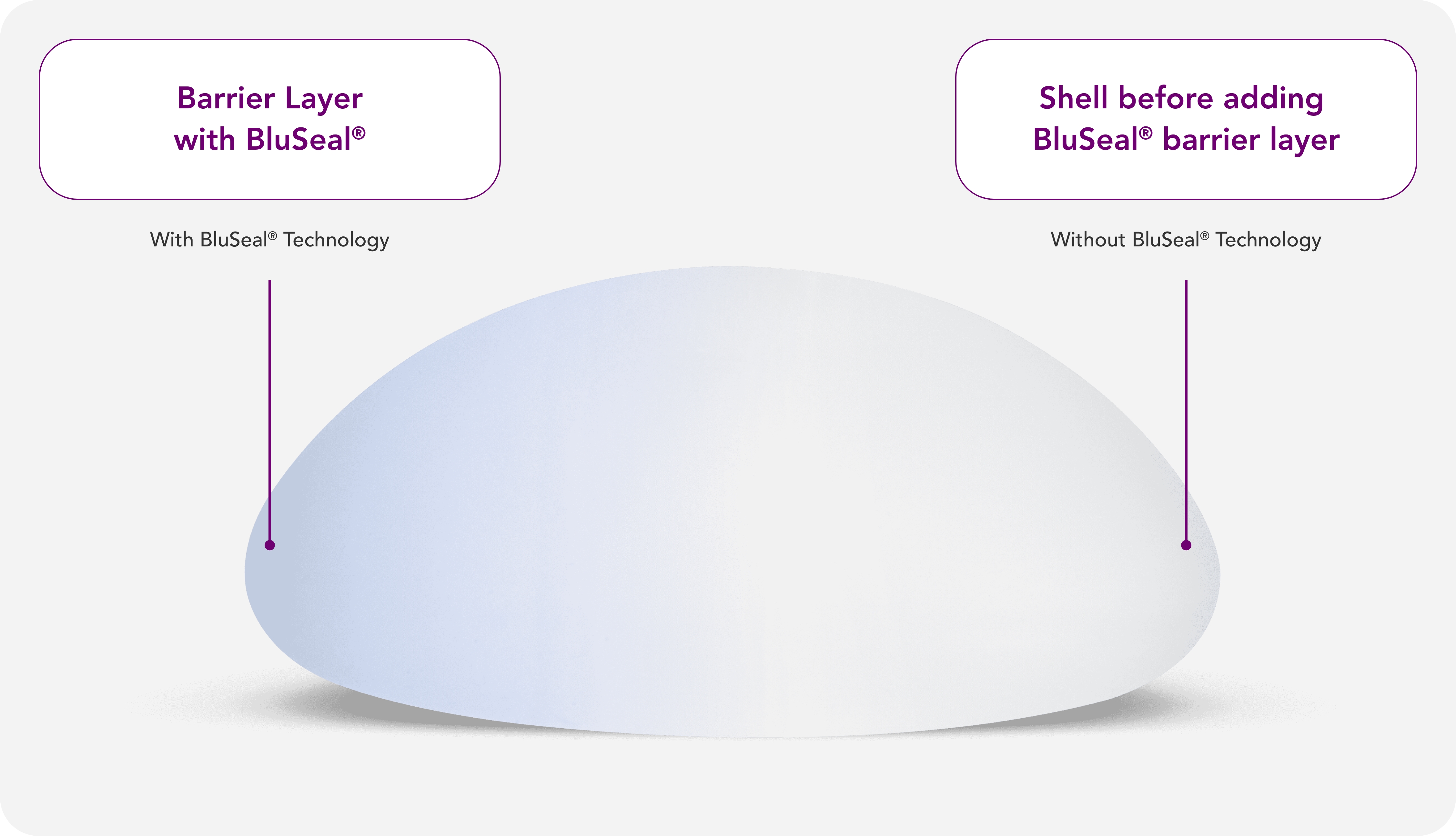
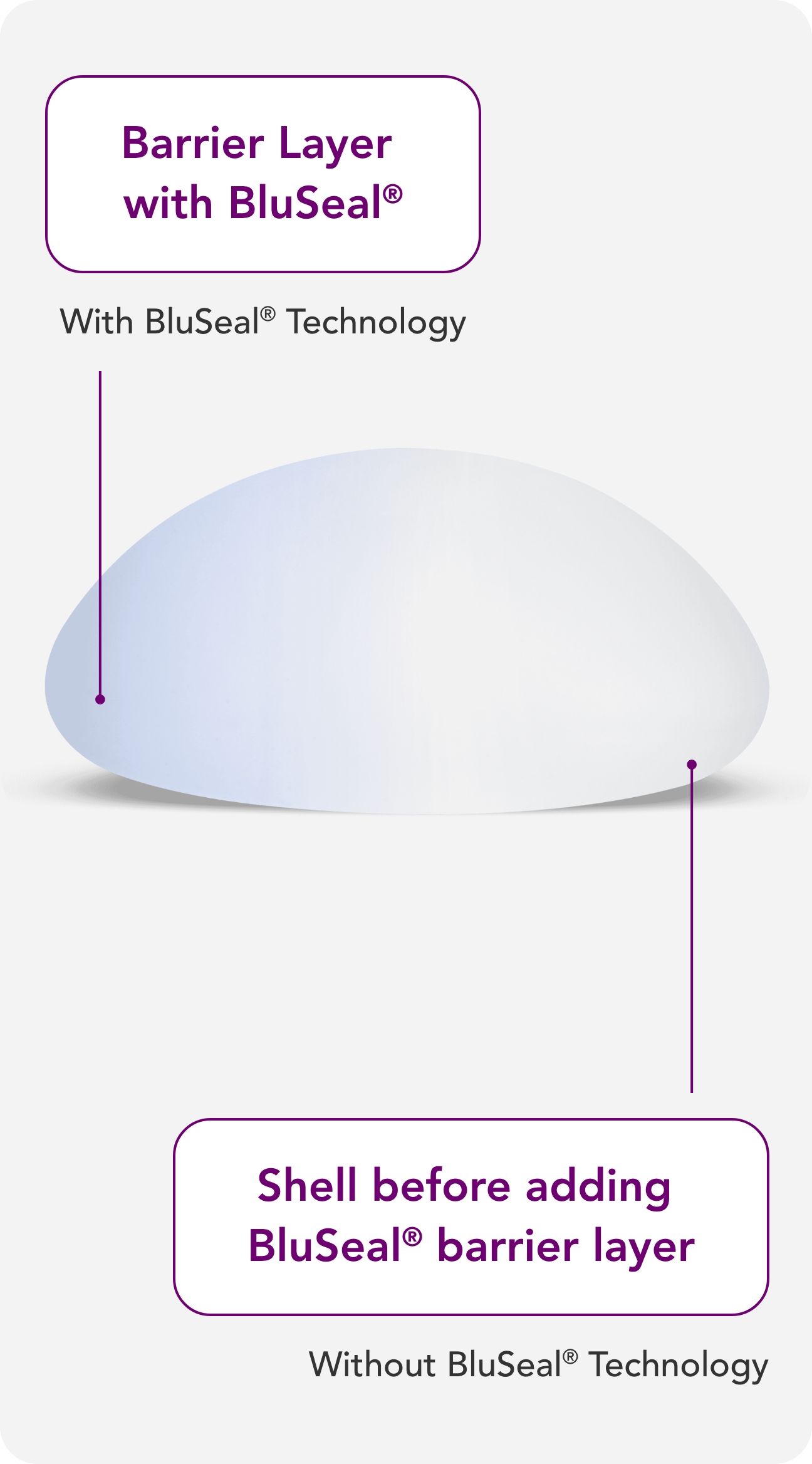
With BluSeal®, Motiva® provides a visual indicator of the barrier layer that confirms the integrity of the external and internal components of the implant, designed for quality assurance and patient safety.5,8
Safety Above Industry Standards
Over 14+ years of low complication rates worldwide.6
-
0.5%
Capsular Contracture4,5*
(when the scar tissue surrounding the implant tightens or hardens).
-
0.6%
Rupture4,5*
(when the implant shell has a tear or hole).
-
0.9%
Infection4,5*
(when microorganisms overgrow in the body).
*Primary Augmentation Cohort, 4-Year CORE Study
References
1. Doloff JC, Veiseh O, de Mezerville R, et al. The surface topography of silicone breast implants mediates the foreign body response in mice, rabbits and humans. Nature Biomedical Engineering. 2021. doi:10.1038/s41551-021-00739-4
2. Jones P, Mempin M, Hu H, et al. The functional influence of breast implant outer shell morphology on bacterial attachment and growth. Plast Reconstr Surg. 2018;142(4):837-849. doi: 10.1097/PRS.0000000000004801
3. James G, Boegli L, Hancock J et al. Bacterial Adhesion and Biofilm Formation on Textured Breast Implant Shell Materials. Aesth Plast Surg. 2019 Apr; 43:490–497. doi: 10.1007/s00266-018-1234-7
4. Establishment Labs® Holdings Corp. Establishment Labs® Notes Presentation of 4-Year Results from Motiva® U.S. IDE Study at The Aesthetic Meeting 2024. https://investors.establishmentlabs.com/press-releases/pressreleasesdet… May 2nd, 2024. Accessed May 28th, 2024.
5. Aitzetmuller-Kleitz ML, Yang S, Wiebinghaus P, Wellenbrock S, Ozturk M, Kuckelhaus M et al. Complication rates after breast surgery with the Motiva Smooth SilkSurface silicone gel implants – A systematic review and meta-analysis. Clin. Med. 2023, 12,1881. doi: 10.3390/jcm12051881
6. Establishment Labs®, Post-Market Surveillance Preliminary Results Q1 2024. Internal Data on File.
7. Establishment Labs, TR-001038: Rheological analysis of silicone filling gels of Motiva Implants® and other brands’ silicone filling gels using the BTC-2000. Data on File.
8. Establishment Labs, TSD-001019: Technical Summary Document Assessment of silicone diffusion/ gel bleed from Motiva Implants®, according to ISO 14607:2018 method. Data on File.
9. Glicksman C, Wolfe A, McGuire P. The Study of the Safety and Effectiveness of Motiva SmoothSilk Silicone Gel-Filled Breast Implants in Patients Undergoing Primary and Revisional Breast Augmentation: Three-Year Clinical Data. Aesthet Surg J. 2024 Sep 27:sjae134. doi: 10.1093/asj/sjae134. Epub ahead of print. PMID: 39331509.
Disclaimer
The sale and distribution of this device is restricted to users and/or user facilities that provide information to patients about the risks and benefits of this device in the form and manner specified in the approved labeling provided by Establishment Labs® and Motiva® USA. Federal (USA) Law restricts this device to sale by or on the order of a physician.
The Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast Implants are indicated for breast augmentation for women of at least 22 years old. Breast augmentation includes primary breast surgery to increase the breast size, as well as revision surgery to correct or improve the result of an original primary breast augmentation surgery (i.e., revision-augmentation). Breast Implant surgery is contraindicated in women with active infection anywhere in their bodies, with existing cancer or pre-cancer of their breast who have not received sufficient treatment for those conditions, or who are currently pregnant or nursing. Adequate studies have not been performed to confirm the safety of breast implant surgery in women with these conditions or under these circumstances; therefore, if you have any of the conditions or circumstances listed above, breast augmentation surgery with implants should not be performed at this time. Failure to take into consideration these contraindications may increase the risks involved with breast implant surgery and have the potential to cause harm. Patients should be advised that key complications have historically been associated with silicone gel breast surgery and implantation of silicone gel breast implants including, but not limited to, capsular contracture, implant removal, reoperation, infection, and rupture. Further, breast implants are not lifetime devices and patients should visit their healthcare professional, as recommended. For more detailed information about the benefits and risks of Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast Implants, please visit: www.motivausa.com or call Motiva at 1-800-924-5072.
Motiva®, Establishment Labs®, SmoothSilk®, Ergonomix®, ProgressiveGel Ultima®, Motiva Implants®, ProgressiveGel®, BluSeal® are trademarks of Establishment Labs Holdings® Inc.